Bohr Model Of An Element
Bohr Models First Elements in the Periodic Table.
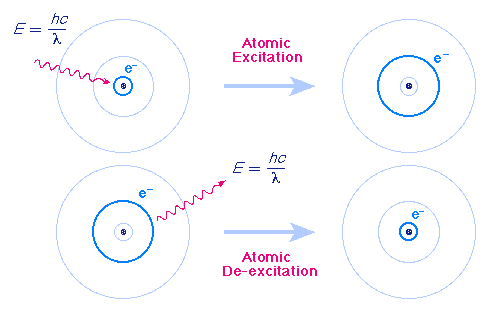
Bohr model of an element. Bohr's Model In 1913, a Danish physicist, Niels Bohr (15–1962;. Bohr found that an electron located away from the nucleus has more energy, and electrons close to the nucleus have less energy. Using the Bohr model, determine the lowest possible energy, in joules, for the electron in the Li 2+ ion.
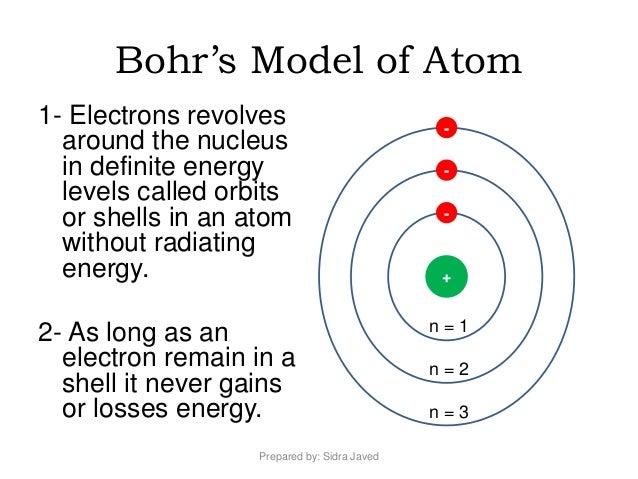
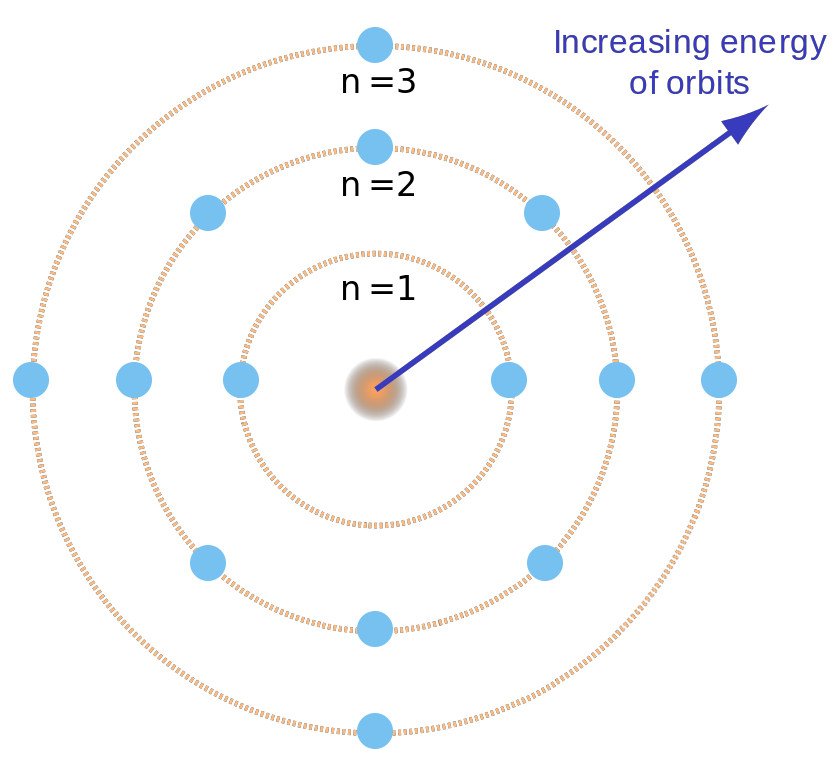
The Bohr model is used to account for the spectrum of the hydrogen atom, but the basic idea is the same for all elements. The second has two 2s and six 2p electrons (8 total);. The number of rings in the Bohr model of an element is determined by row number on the periodic table.
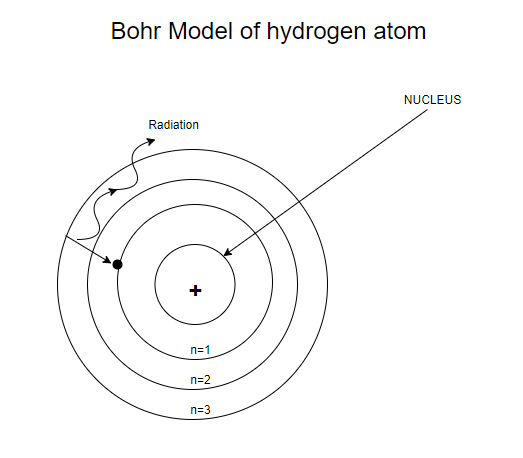
A Bohr diagram can be used to visually show the Bohr model of a particular atom. He was struggling to make sense of all of this. These orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the various shells.
Here's a closer look at the Bohr Model, which is sometimes called the Rutherford-Bohr Model. Now its just used for scaring students. 6th - 9th grade.
Bohr’s model required only one assumption:. BOHR’S ATOMIC MODEL & ITS APPLICATIONS Chapter # 2 Atomic Structure Prepared by:. The Fundamental Particles Particle Charge (Coulomb) Relative Charge Mass (Kg) Mass (a.m.u) Found in:.
Emission spectrum of hydrogen. The third has two 3s electrons,. The Rutherford-Bohr model and van den Broek.
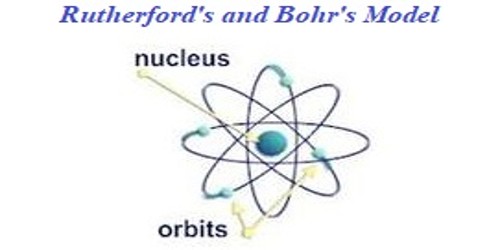
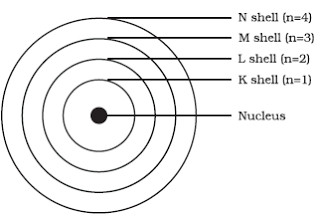
The Bohr model of the atom, a radical departure from earlier, classical descriptions, was the first that incorporated quantum theory and was the predecessor of wholly quantum-mechanical models. The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom model. Niels Bohr knew that Rutherford’s model of the atom was not accurate.
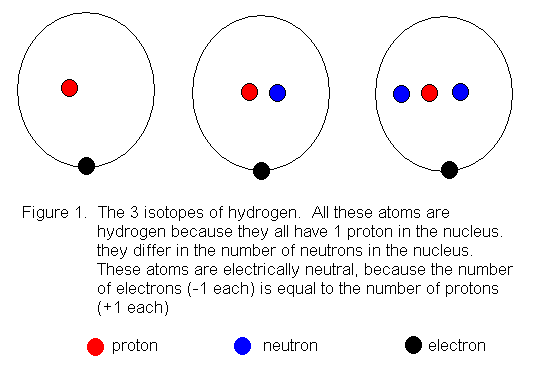
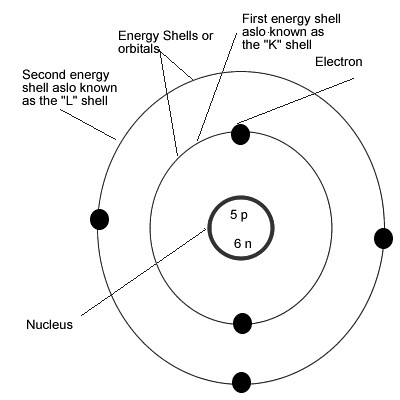
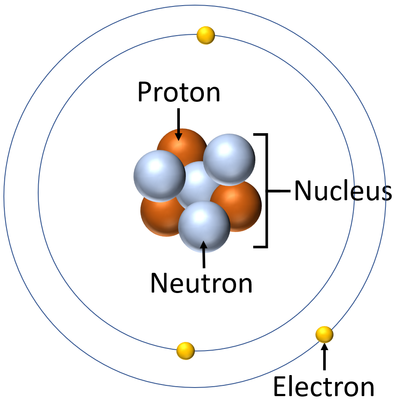
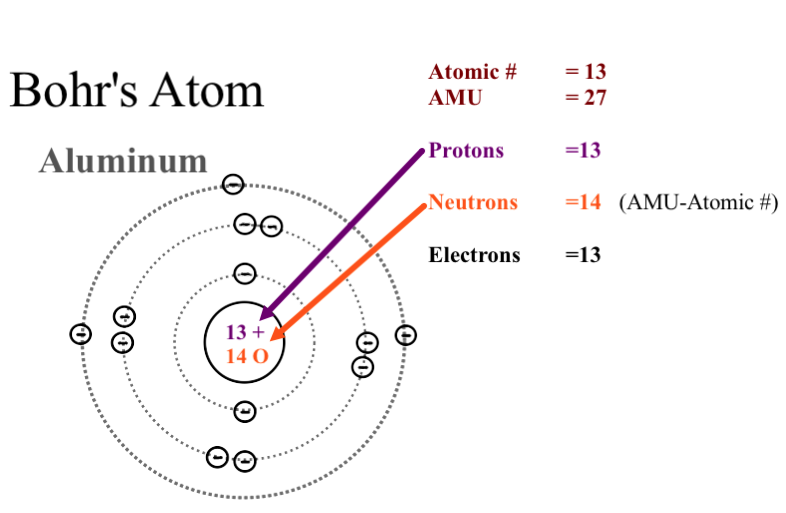
Bohr Model Of Argon Atom Potassium Atom, Copper Atom, Atom Model Project, Bohr. The Bohr model shows the atom as a central nucleus containing protons and neutrons with the electrons in circular orbitals at specific distances from the nucleus (Figure 1 ). –The number of rings increases as you move DOWN the Periodic Table.
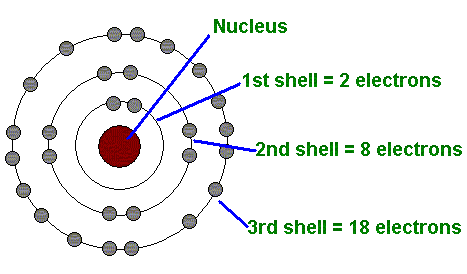
Bohr Atomic Model Cards for First Elements Created Date:. 4) The 2nd shell can hold up to 8 electrons. The Bohr Model Physicists and chemists studied the nature of the light that was given off when electric currents were passed through tubes containing gaseous elements (hydrogen, helium, neon) and when elements were heated (e.g., sodium, potassium, calcium, etc.) in a flame.
For the best answers, search on this site https://shorturl.im/avc17. Bohr Models and the Periodic Table Practice DRAFT. Bohr's Model and its Postulates.
Choose from 161 different sets of bohr elements flashcards on Quizlet. Proton + 1.602 x 10-19 +1 1.6727 x 10-27 1.0073 Nucleus Neutron 0 0 1.6750 x 10-27 1.0087 Nucleus Electron -1.602 x 10-19 -1 9.1095 x 10-31 5.4858 x 10-4 Outside Nucleus Prepared by. Quantum Mechanics Sheds Light on the Atom:.
Bohr model radii (derivation using physics) Up Next. The electron moves around the nucleus in circular orbits that can have only certain allowed radii. The Bohr diagram for boron shows a central nucleus containing five protons.
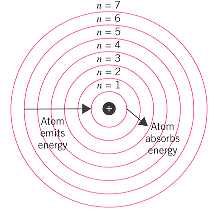
As Bohr had noticed, the radius of the n = 1 orbit is approximately the same size as an atom. Learn bohr models periodic table with free interactive flashcards. In the Bohr model of the atom, what is the maximum number of electrons in the first shell?.
This central charge would thus be approximately half the atomic weight. A Bohr diagram can be used to visually show the Bohr model of a particular atom. Bohr’s Model of an Atom Bohr’s model consists of a small nucleus (positively charged) surrounded by negative electrons moving around the nucleus in orbits.
As was mentioned before, a neutral Phosphorus Atom contains five valence electrons in the third shell. Essentially each energy level can hold a different number of electrons. Niels Bohr, a Danish scientist, explained this line spectrum while developing a model for the atom:.
The Bohr model of hydrogen is a semi-classical model because it combines the classical concept of electron orbits with the new concept of quantization. 6th - 9th grade. Bohr models are insanely complex and only have been dont for hydrogen and helium.
Work was done on lithium, but it was too complex to be solved, then the quantum theory came along, and the bohr theory was abandoned. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. The Bohr model was later superseded by the Sommerfield model.
See more ideas about Bohr model, Atom, Chemistry. Nobel Prize in Physics, 1922), proposed a theoretical model for the hydrogen atom that explained its emission spectrum. Key features of the Bohr model include set orbits for electrons around the nucleus, as well as the ability of electrons to move from one orbit to another.
An atom is the smallest building block of an element and each element has a specific number of subatomic particles. In this case, an electron orbiting the nucleus would have a centripetal force, F, equal to:. Learn bohr elements with free interactive flashcards.
It needed slight modifications. You can see a Bohr Model of Phosphorus below this. Bohr Model of Copper.
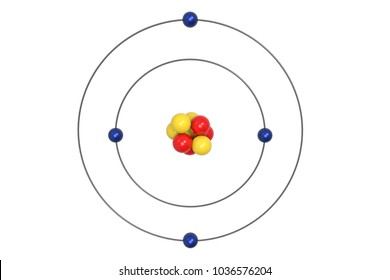
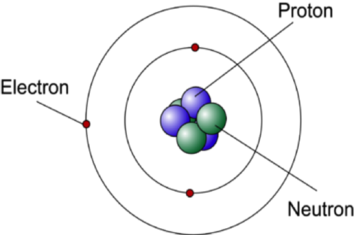
The Bohr Model has an atom consisting of a small, positively charged nucleus orbited by negatively charged electrons. 2) You should have 6 total electrons for Carbon. The first energy level has two 1s electrons (2 total);.
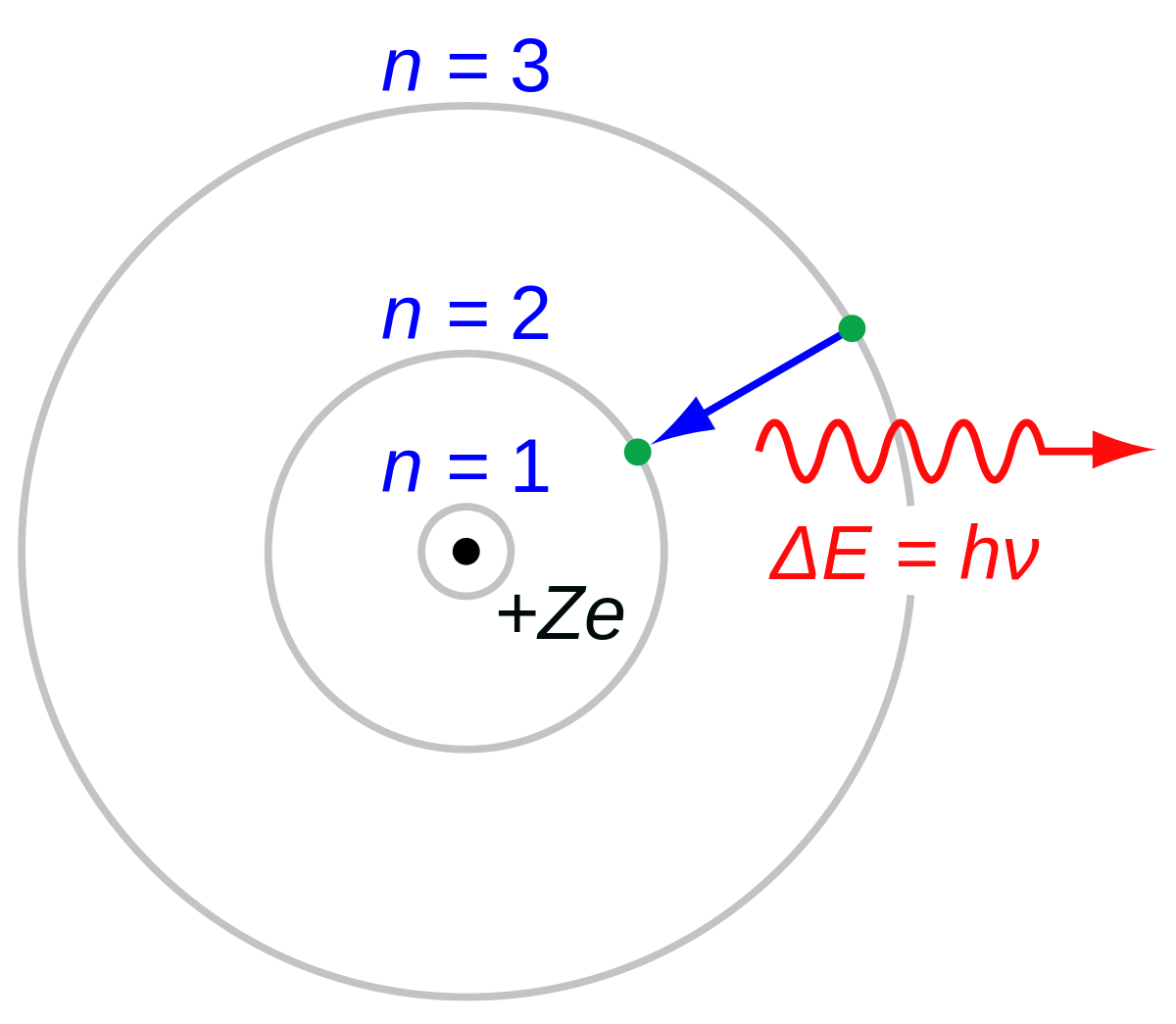
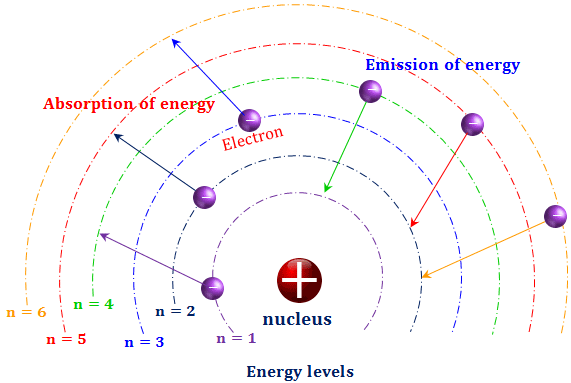
–Elements in the same row have the same number of rings. Energy of an Electron in nth Orbit. Bohr used Max Planck and Albert Einstein’s findings along with his understanding of emission and absorption spectra of chemical elements to develop his model of the atom.
You can use craft cotton (or Styrofoam TM) balls, poster board, compass, glue, and string to make a model of a boron atom. In the Bohr model, electrons are assumed to exist in fixed orbits around the nucleus of the atom. Sodium (Na) and Magnesium (Mg) are.
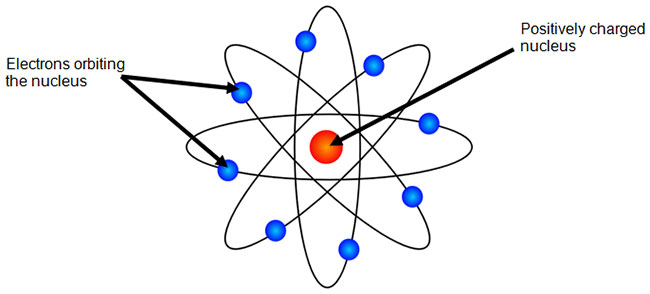
What is the bohr model diagram for phosphorus answers == the phosphorus bohr model has 3 shells because it has 15protons and 16 neutrons 30 97 is about 31 so 31 15protons = 16 neutrons the number of how to draw bohr rutherford diagrams phosphorous how to draw the bohr rutherford diagram for phosphorous 2 electrons can go in the first shell 8 in the second 8 in the. Bohr developed the Bohr modelof the atom, in which he proposed that energy levels of electronsare discrete and that the electrons revolve in stable orbits around the atomic nucleusbut can jump from one energy level (or orbit) to another. Bohr Model of Phosphorus Neon Atom Model, 5th Grade Science Projects, 8th copper bohr diagram wedocable - 28 images - copper element protons and.
Using the Bohr model, determine the lowest possible energy, in joules, for the electron in the Li 2+ ion. A google search can answer that. Bohr models are used to predict reactivity in elements.
Overview of the Bohr Model Niels Bohr proposed the Bohr Model of the Atom in 1915. Although the Bohr model has been supplanted by other models, its underlying principles remain valid. Bohr assumed that Rutherford’s ideas of the electrons orbiting the nucleus were correct.
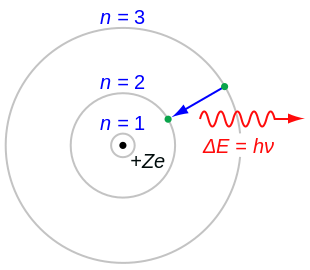
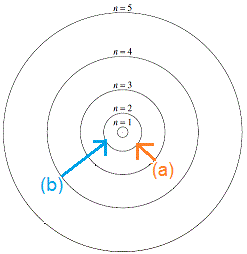
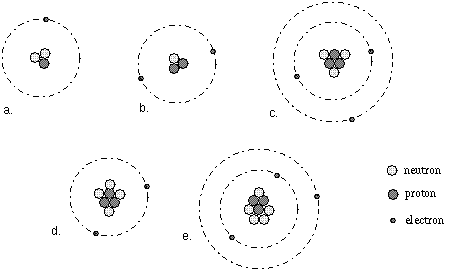
To draw a Bohr model of an atom, first find the number of protons, neutrons and electrons in the atom from its atomic weight and atomic number. In other words, electrons are fixed at a distance, r, from the nucleus. The single electron in hydrogen revolves around the nucleus in one of a limited number of circular orbits.
An early model of the atom was developed in 1913 by Danish scientist Niels Bohr (15–1962). –The row number tells you how many energy levels, or rings, the element has. An atom is the main component of an element and the determinant of an element.
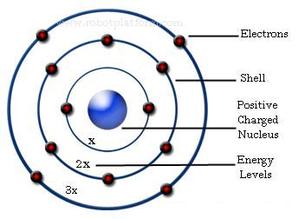
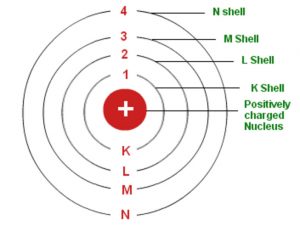
The remarkable success of this model prompted many physicists to seek an explanation for why such a model should work at all, and to seek an understanding of the physics behind the postulates of. Bohr used the term energy levels (or shells) to describe these orbits of differing energy. A Bohr model is a way of visually depicting the structure of an atom of a particular element.
Dec 16, 14 - This board is about Bohr's idea of what a close-up atom looks like. K - 12th grade. When looking at Bohr models, we look at its valence electrons (the electrons on the last energy level) to determine reactivity.
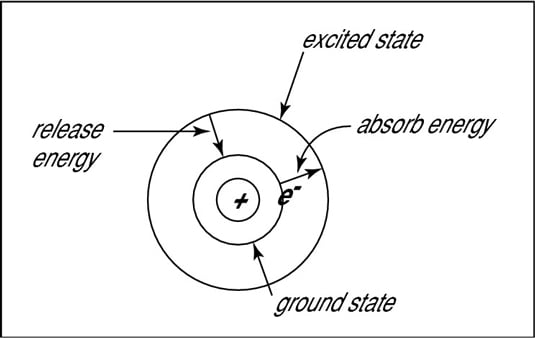
3) Only two electrons can fit in the 1st shell. When it is in the orbit closest to the nucleus it is in its ground state, this electron is in the valence band of the. Bohr Diagrams 1) Check your work.
Bohr model, description of the structure of atoms, especially that of hydrogen, proposed (1913) by the Danish physicist Niels Bohr. Before drawing a Bohr Model… •Horizontal rows on the Periodic Table are called periods. Using the Main Group Elements of the Periodic Table to Draw Bohr-Rutherford Diagrams He.
Rutherford's model of an Atom was undoubtedly a breakthrough in Atomic studies. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. Enjoy the videos and music you love, upload original content, and share it all with friends, family, and the world on YouTube.
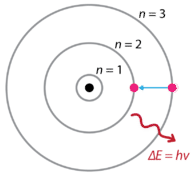
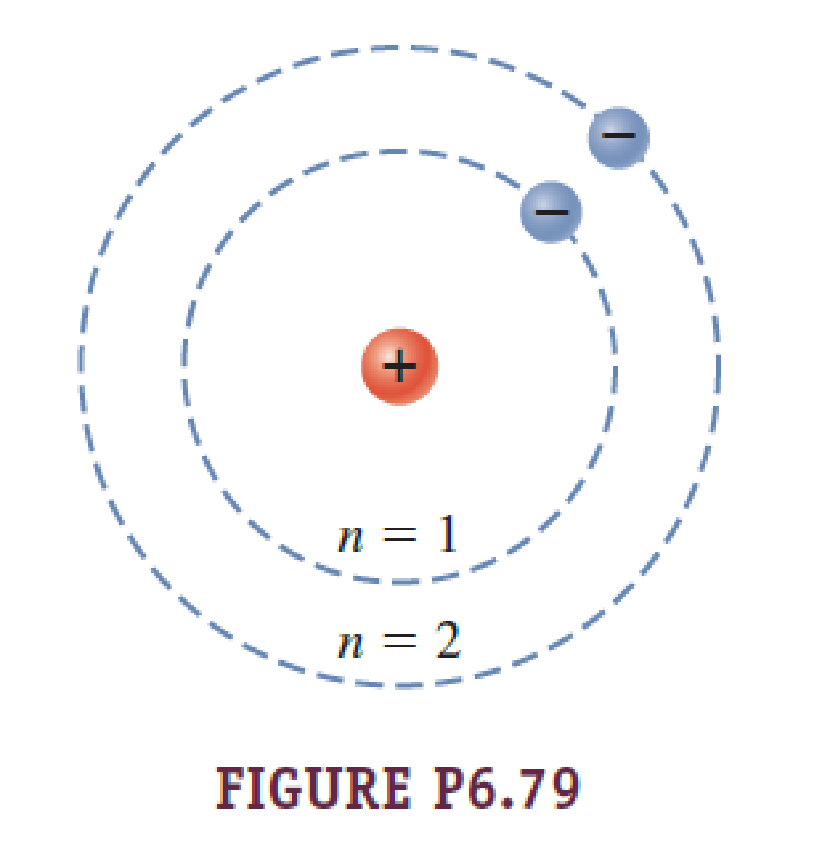
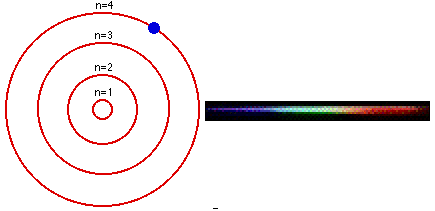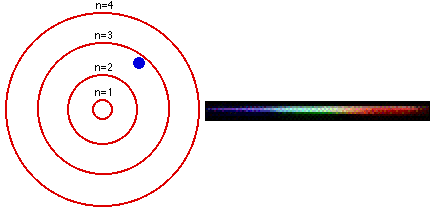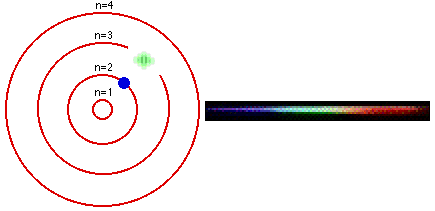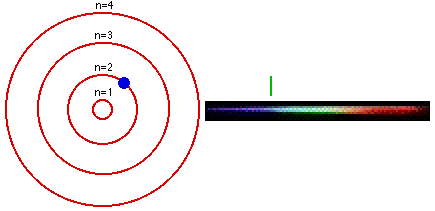
Group 18 elements (helium, neon, and argon are shown) have a full outer. The Bohr Model DRAFT. Using the Bohr model, determine the energy, in electron volts, of the photon produced when an electron in a hydrogen atom moves from the orbit with n = 5 to the orbit with n = 2.
In 1911, Ernest Rutherford gave a model of the atom in which a central nucleus held most of the atom's mass and a positive charge which, in units of the electron's charge, was to be approximately equal to half of the atom's atomic weight, expressed in numbers of hydrogen atoms. For a given element, the atomic number indicates the number of _____ Preview this quiz on Quizizz. Students may use this board as a resource for studying and informational purposes.
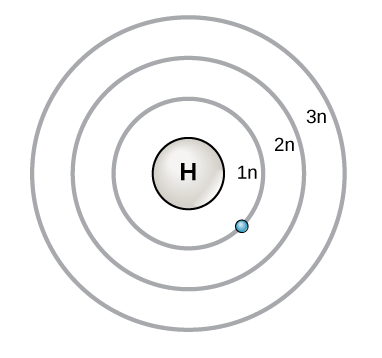
Bohr diagrams show electrons orbiting the nucleus of an atom In the Bohr model, electrons are pictured as traveling in circles at different shells, Each element, when electrically neutral, has a number of electrons For example, the 1n shell represents the first energy level located closest to the nucleus.Now offering rare physics books for. After that, place the neutrons and the protons in the nucleus, and draw the electrons in their designated shells. Bohr model in atomic physics the rutherford–bohr model or bohr model or bohr diagram introduced by niels bohr and ernest rutherford in 1913 depicts the atom as a chemical elements an interactive periodic table of an up to date periodic table with detailed but easy to understand information.
A document on this topic may be found here. In Bohr’s model, radius an of the orbit n is given by the formula an = h2n2 ε 0 /π 2, where ε 0 is the electric constant. However, it wasn't completely correct.
Since the Bohr model is a quantum-physics-based modification of the Rutherford model, many sources combine the two:. Bohr Diagram for Phosphorus. Visit chemical elements, crystals, melting points,Bohr Model of Copper.
The Bohr model shows that the electrons in atoms are in orbits of differing energy around the nucleus (think of planets orbiting around the sun). What element is this?. Neils Bohr knew about all of these facts, and in the early part of the century was collaborating with Rutherford.
Bohr's Model of atom. Reactivity refers to how likely an element is to form a compound with another element. Postulates of Bohr’s Model of an Atom.
The Bohr model was an improvement on the earlier cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911). What element does this Bohr model represent?. Choose from 500 different sets of bohr models periodic table flashcards on Quizlet.
Learn how to use an element's position on the periodic table to predict its properties, electron configuration, and reactivity. Bohr diagrams indicate how many electrons fill each principal shell. It corresponds to principal quantum number n.
Bohr model energy levels (derivation using physics) Bohr model energy levels. Using the Bohr model, determine the energy, in electron volts, of the photon produced when an electron in a hydrogen atom moves from the orbit with n = 5 to the orbit with n = 2. He also knew about the existence of line spectra from chemical elements;.
Democritus Model – 00 yrs ago Dalton Model - 1803 Thomson Model - 17 Rutherford Model - 1908 Bohr Model - 1913 Wave Model - Modern Name The Atomic Model of Matter On the graphic organizer provided summarize and illustrate the features of the atom for each model, and state the problem with each model.
Atomic Structure The Bohr Model Dummies

Problems With Bohr S Model Of The Atom

Bohr S Atomic Model Chemistrygod
Bohr Model Of An Element のギャラリー

Cbse Ncert Solutions For Class 11 Chemistry Bohr S Model Of Atom
Q Tbn 3aand9gcqax Gsl Awasluaqiawema3yzmhh8ibqkidw Usqp Cau
Q Tbn 3aand9gcseyobbyyxbjn6tvzukgg8pqxexr4l9yuq G9cwemw2k9xszngu Usqp Cau

Atom Bohr S Shell Model Britannica

The Open Door Web Site Ib Physics Atomic And Nuclear Physics The Bohr Model Of The Hydrogen Atom
Write Bohrs Postulates For The Hydrogen Atom Model Class 10 Physics Cbse
Describe Comparison Between Rutherford S And Bohr S Model Qs Study
Electronic Structure And Chemical Bonding
3
Bohr Model Wikipedia
Bohr Model Atom Electron Configuration Argon Calcium Png 1024x1024px Bohr Model Aluminium Area Argon Atom Download
Niels Bohr The History Of The Atom

The Bohr Model Of The Atom Ck 12 Foundation

Oxygen Atom Bohr Model With Proton Neutron And Electron 3d Illustration Bohr Model Atom Model Atom Model Project
Boron2 The Bohr Rutherford Model Helium Atom Atomic The Flickr

Bohr Atomic Model
Niels Bohr
Bohr Model Images Stock Photos Vectors Shutterstock

Bohr S Model

Bohr S Model For Hydrogen Atom

Bohr Model Of First 18 Elements Of The Periodic Table 11 Download Scientific Diagram
Q Tbn 3aand9gcsmaz 1eyztdqbvapeouj0fznnxyysqsglrb8j4ou2j8icjvqgp Usqp Cau

What Is Bohr S Model Of Neon Quora
Q Tbn 3aand9gcrpthnrdnqo6wkegnasu6udb Vx1mltulbdahtujzw4kigjpw E Usqp Cau

Draw A Sketch Of Bohr S Model Of An Atom With Three Shells

Bohr S Model Of An Atom With Postulates And Limitations Byju S
Bohr Model High Resolution Stock Photography And Images Alamy
Bohr Model Key Stage Wiki
Bohr S Model Of An Atom Chemistry Class 11 Structure Of Atom
The Bohr Model

Bohr Model Definition Equation Study Com

Bohr Models Why Bohr Models 1 Bohr Models Are Used To Predict Reactivity In Elements 2 Reactivity Refers To How Likely An Element Is To Form A Compound Ppt Download

Bohr Model Of The Atom
Bohr Model Wikipedia
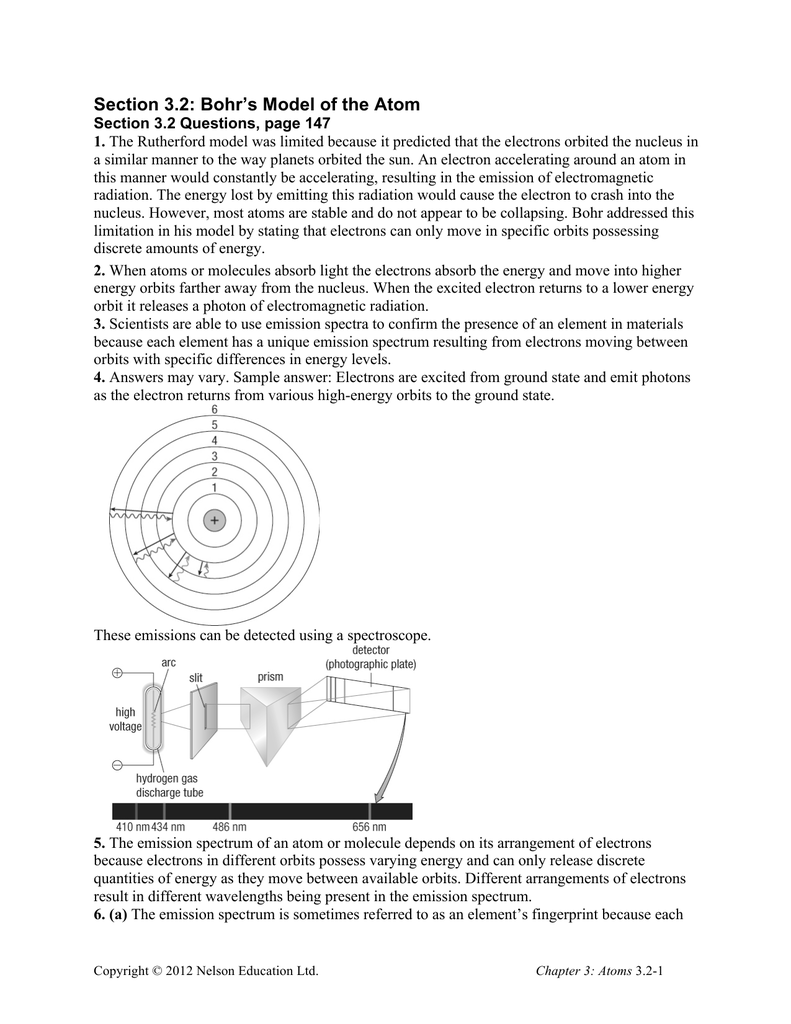
Section 3 2 Bohr S Model Of The Atom
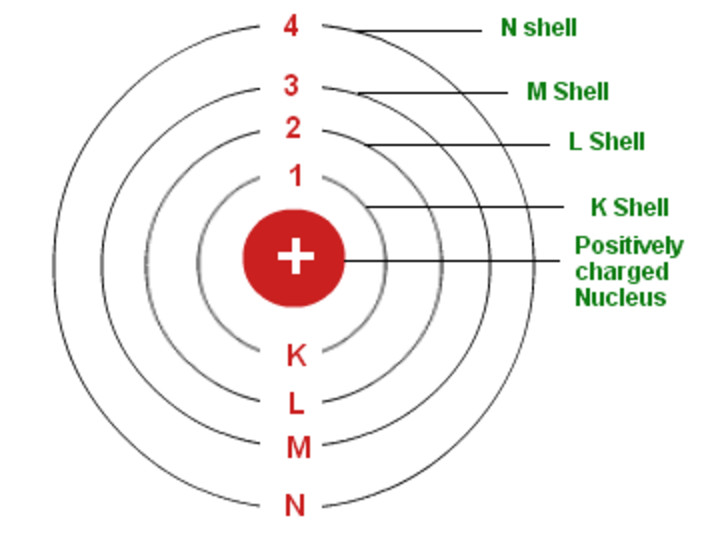
Bohr S Model Of An Atom Chemistry Class 11 Structure Of Atom

Using The Aph Periodic Table To Determine Valence Electrons Perkins Elearning

How To Draw Bohr Models Youtube

Atom Illustration Bohr Model Sodium Atom Chemistry Rutherford Model Copper Shell Miscellaneous Chemical Element Electron Png Pngwing
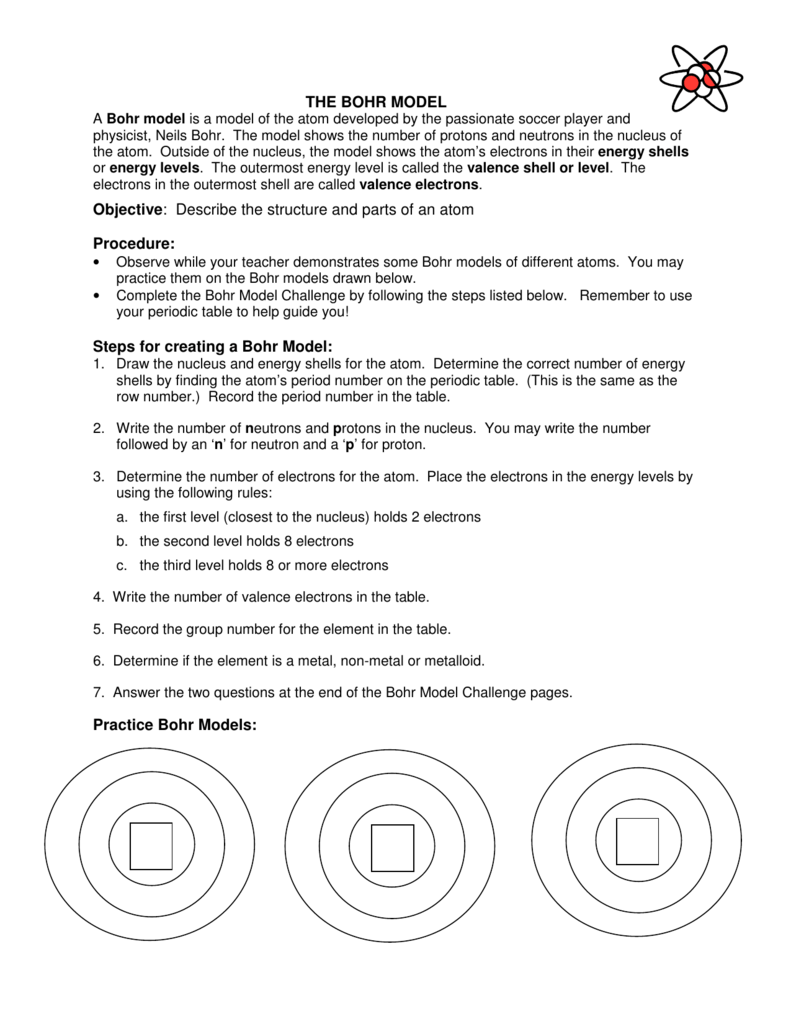
Steps For Creating A Bohr Model Cohick

Sc Element Gallery For Scandium Element Bohr Model Atom Diagram Bohr Model Electron Configuration

Bohr Model Of First 18 Elements Of The Periodic Table 11 Download Scientific Diagram
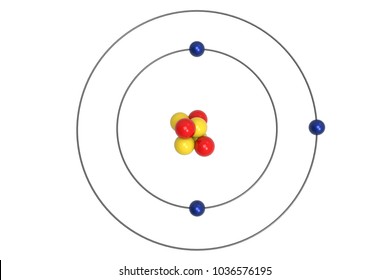
Bohr Model Images Stock Photos Vectors Shutterstock
The Number Of Rings In The Bohr Model Of Any Element Is Determined By What Socratic
Bohr Model Study Material For Iit Jee Askiitians
Bohr Model Of The Atom Overview And Examples
Bohr Model Of Atomic Structure Chemistry Tutorial
5 6 Bohr S Atomic Model Chemistry Libretexts
Niel Bohr S Atomic Theory Explained Science Abc
The Radius Of Circular Electron Orbits In The Bohr Model Of The Hydrogen Atom Are Given By 5 29 10 11 M N 2 Where N Is The Electron S Energy Level

Which Of The Following Do Not Represent Bohr S Model Of An Atom Correctly

Bohr Model Bohr Atomic Model Chemistry Tutorcircle Com Bohr Model Chemistry Projects Science Projects

Bohr Model Description Development Britannica

Bohr Model Of Atom Bohr S Postulates
Bohr Model Of Atomic Structure Chemistry Tutorial

Bohr S Model Of Hydrogen Article Khan Academy

Bohr S Theory Of The Hydrogen Atom Physics

What Is Bohr S Atomic Model Universe Today

Bohr S Model Of The Atom Explains Atomic Spectra

Where Did Bohr S Model Of The Atom Fail Quora
Bohr Model Aca Grade 8 Science
Bohr S Model Of The Atom Ppt Video Online Download

Bohr Model Bohr Atomic Model Bohr Model Biology Experiments Atom Model Project
Bohr S Model Of An Atom Class 9 Structure Of An Atom
The Bohr Model Texas Gateway

Video The Bohr Model Of The Atom Nagwa
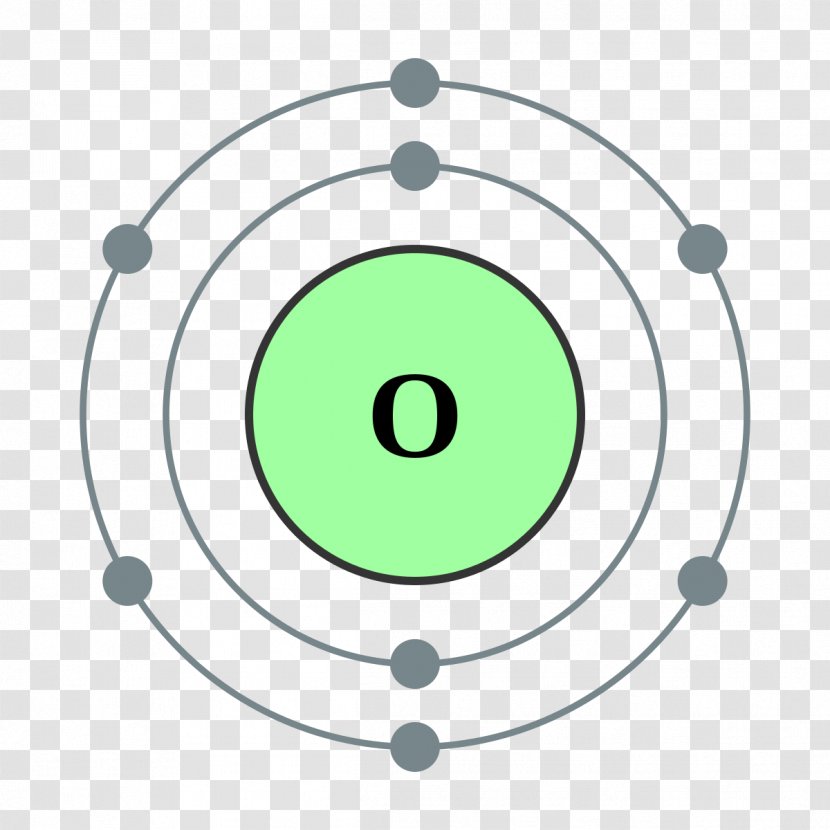
Bohr Model Chemical Element Oxygen Atomic Theory Green Shells Transparent Png
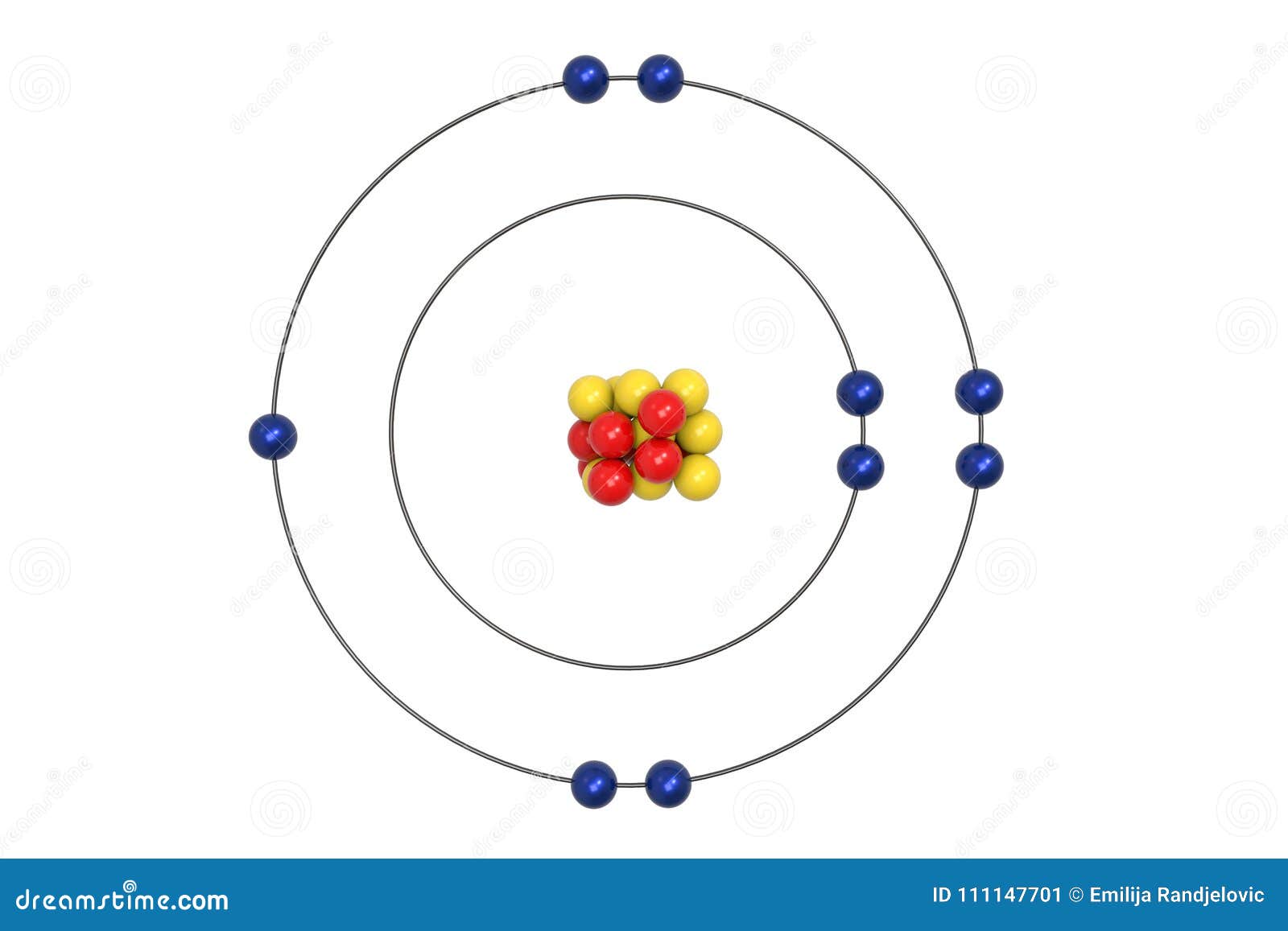
Fluorine Atom Bohr Model With Proton Neutron And Electron Stock Illustration Illustration Of Elements Core
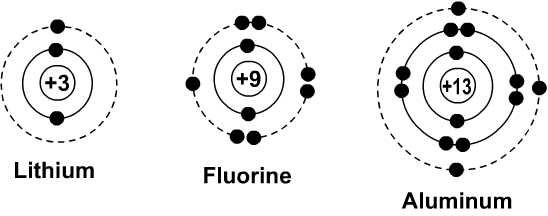
Bohr Diagrams Of Atoms And Ions Chemistry Libretexts

Bohr S Model Of Atom And Equation Chemistrybytes Com
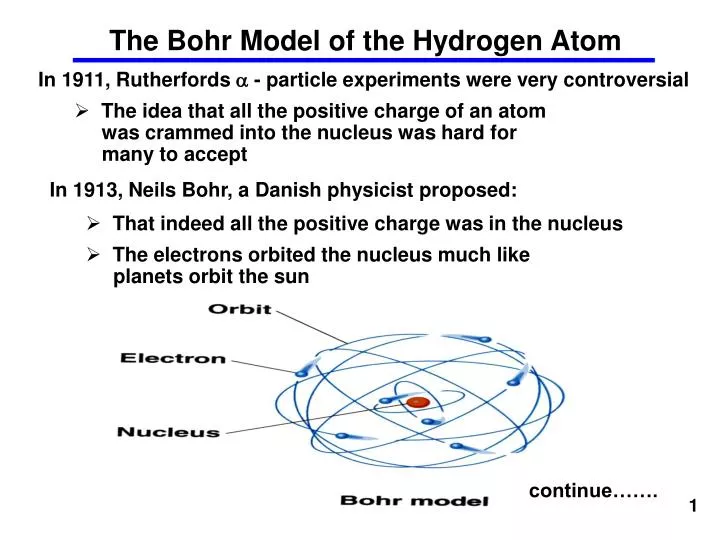
Ppt The Bohr Model Of The Hydrogen Atom Powerpoint Presentation Free Download Id

Features Limitations Of Bohr Model Justscience
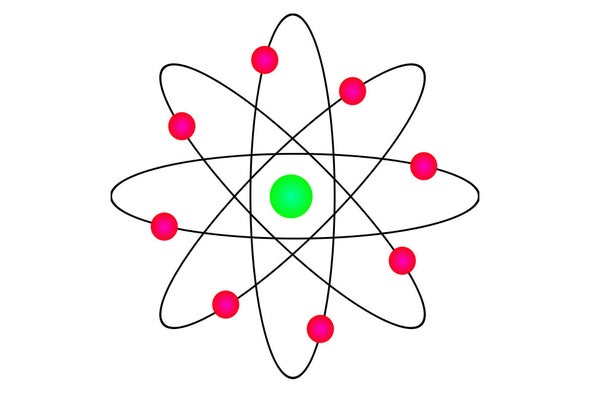
Why It S Okay To Teach Wrong Ideas In Physics Scientific American Blog Network

Bohr S Model Of Atom And Equation Chemistrybytes Com

Bohr S Atomic Model Chemistrygod
How Bohr S Famous Model Of The Atom Was Created Kim Rendfeld
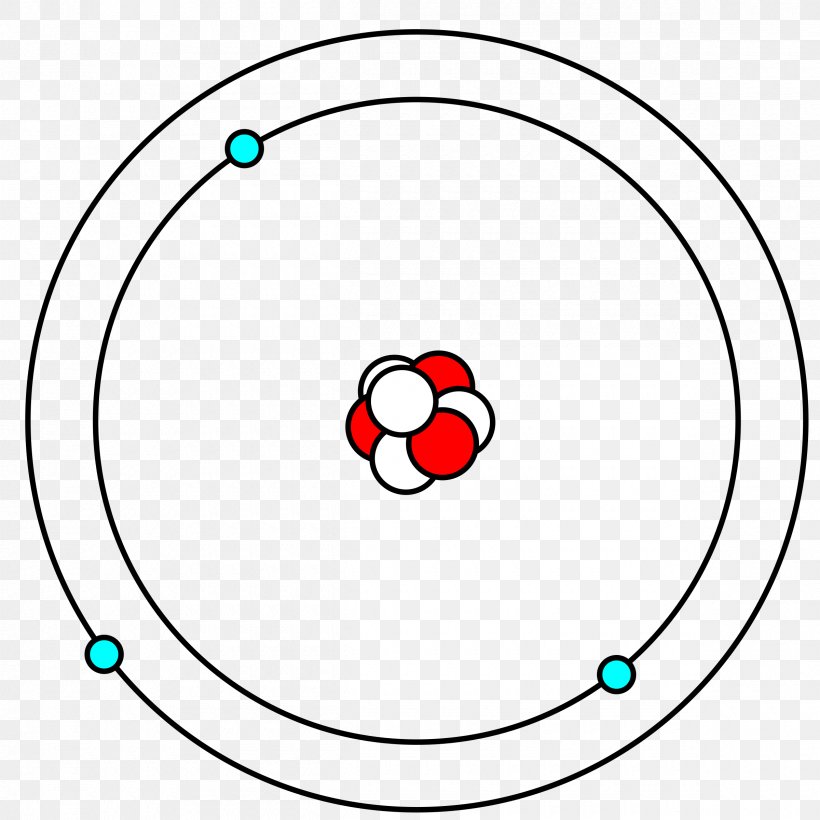
Bohr Model Lithium Atom Lithium Atom Lewis Structure Png 2400x2400px Bohr Model Area Atom Atomic Number
Electromagnetic Radiation
Bohr Diagrams Of Atoms And Ions Chemistry Libretexts
Bohr Model Energy Of Hydrogen Atom Priyamstudycentre

Bohr S Model Of Hydrogen Article Khan Academy
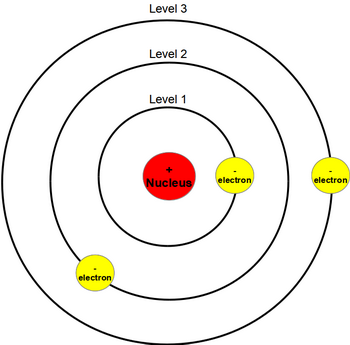
What Does Bohr S Model Of The Atom Look Like Socratic

Bohr S Model Of An Atom Atoms And Molecules Don T Memorise Youtube

Bohr Model Of Atom Bohr S Postulates
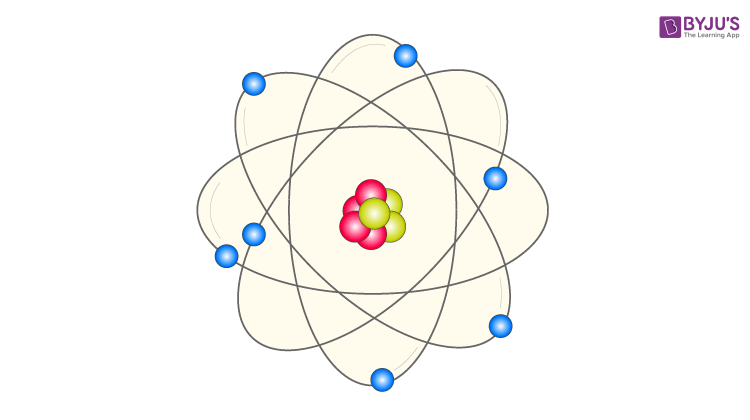
Bohr Model Of The Hydrogen Atom Equation Formula Limitations

Helium Atom Bohr Model Chemistry Helium Miscellaneous Chemical Element Png Pngegg
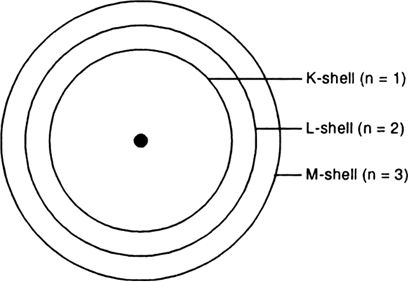
Draw A Sketch Of Bohr S Model Of An Atom With Three Shells From Science Structure Of The Atom Class 9 Himachal Pradesh Board
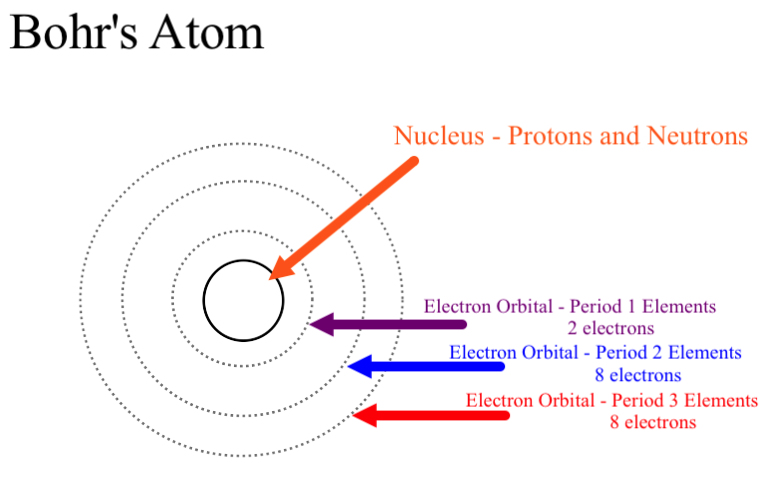
The Number Of Rings In The Bohr Model Of Any Element Is Determined By What Socratic

The Bohr Model Ck 12 Foundation
Q Tbn 3aand9gcrmlcfzprcu4fnzv1sskmhmeayk5haonja3ig Usqp Cau

Chemistry Atom Chlorine Bohr Model Atomic Theory Chemical Element Electron Shell Atomic Number Png Nextpng
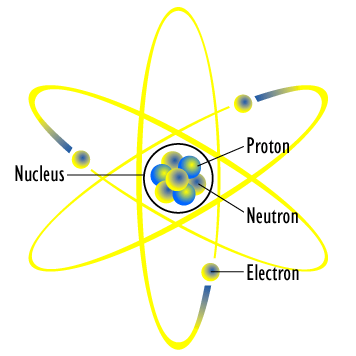
Bohr S Model Of Hydrogen Article Khan Academy
Bohr Model Of The Atom Overview And Examples
Ppt Lecture 15 Bohr Model Of The Atom Powerpoint Presentation Free Download Id
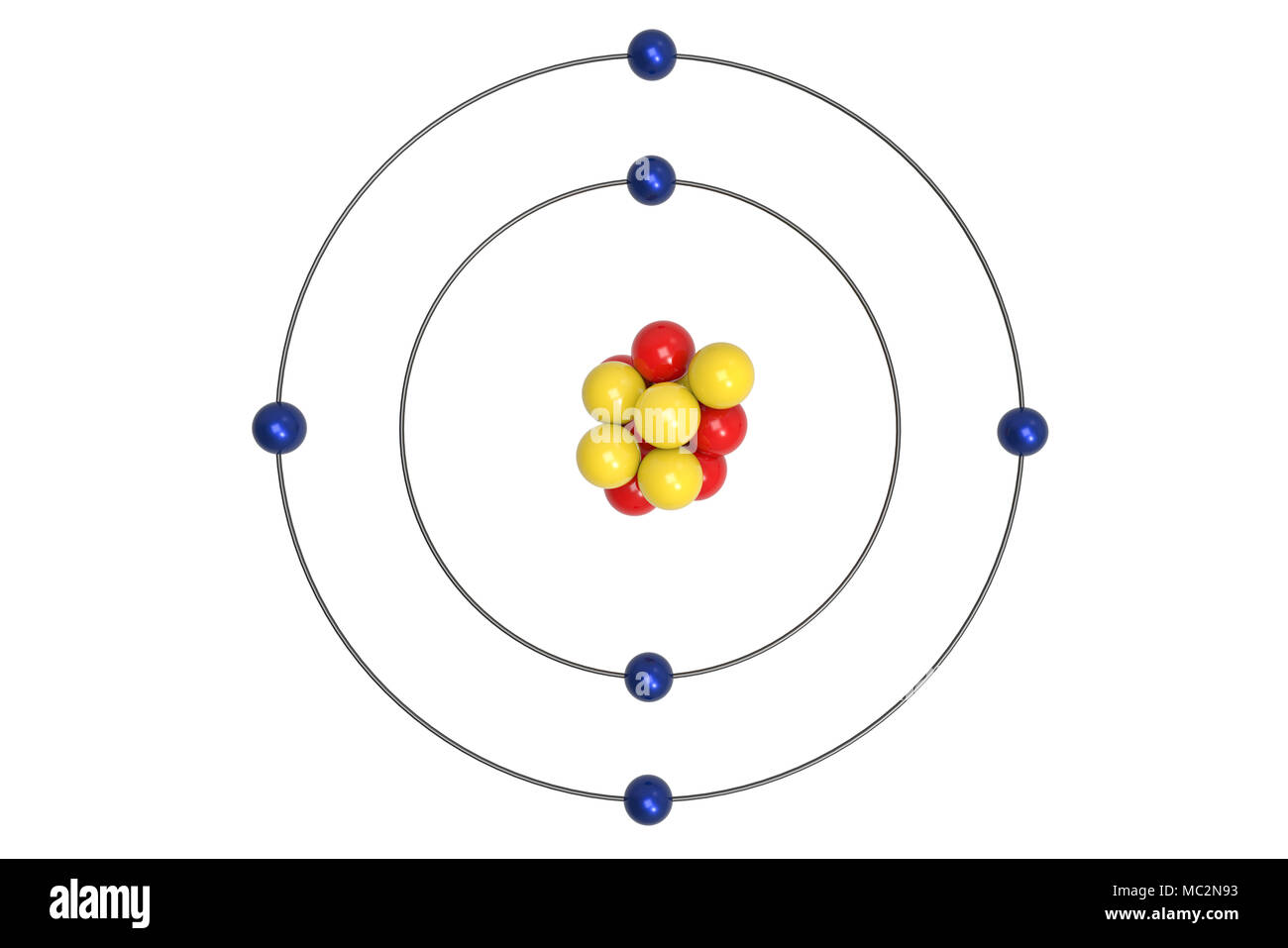
Bohr Model High Resolution Stock Photography And Images Alamy

The Bohr Model Introduction To Chemistry

Bohr S Model Of An Atom With Postulates And Limitations Byju S
The Bohr Model Of The Hydrogen Atom Schooltube Safe Video Sharing And Management For K12

Bohr Model 3d Google Search Bohr Model Science Projects School Projects
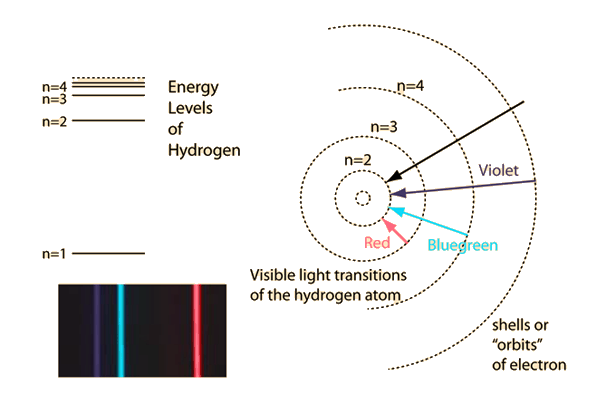
Wave Nature Of Electron
Www Topperlearning Com Answer Draw A Sketch Of Bohr S Model Of An Atom With Atomic Number 15 Fgmaoryyy



