Model Of An Atom Simple
Here's what you need:.
Model of an atom simple. Here we use it to derive the energy levels of hydrogen. It is connected with a fixed value of momentum. This model of the atom is indeed very simple and we can see why there is a geometric regularity to the electron energy shells as the atom is built up.
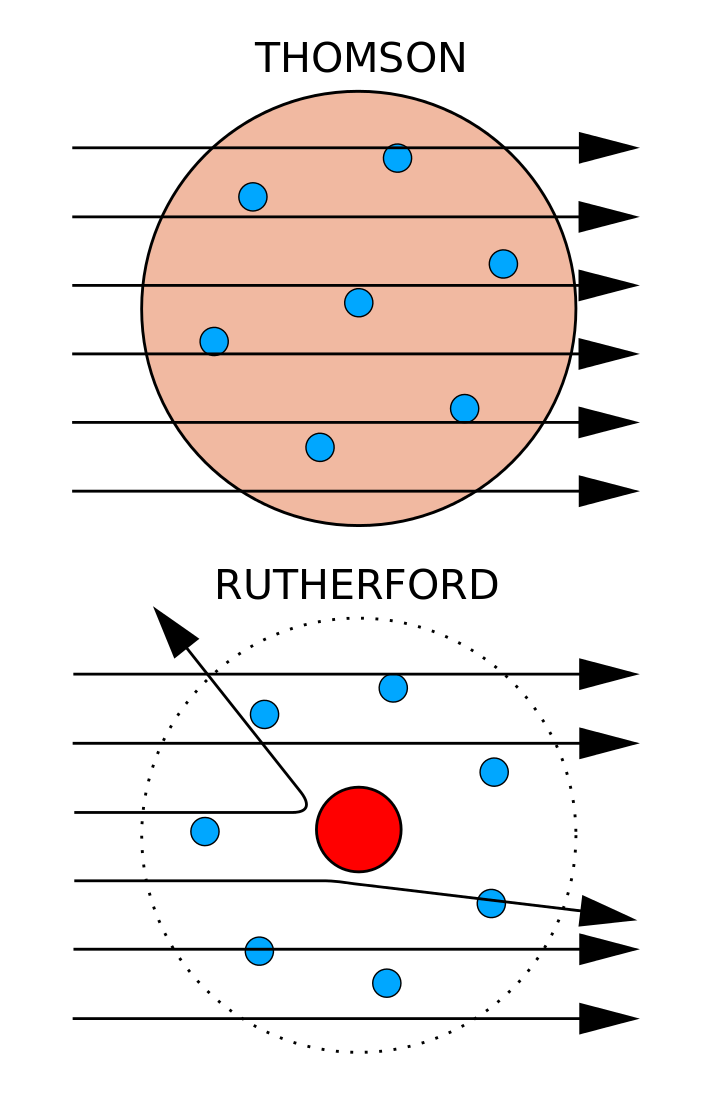
Major space in an atom is empty – A large fraction of α-particles passed through the gold sheet without getting deflected. Develop models to describe the atomic composition of simple molecules and extended structures. The atom is the basic building block for all matter in the universe.
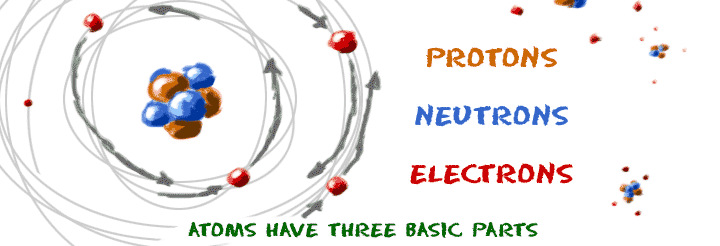
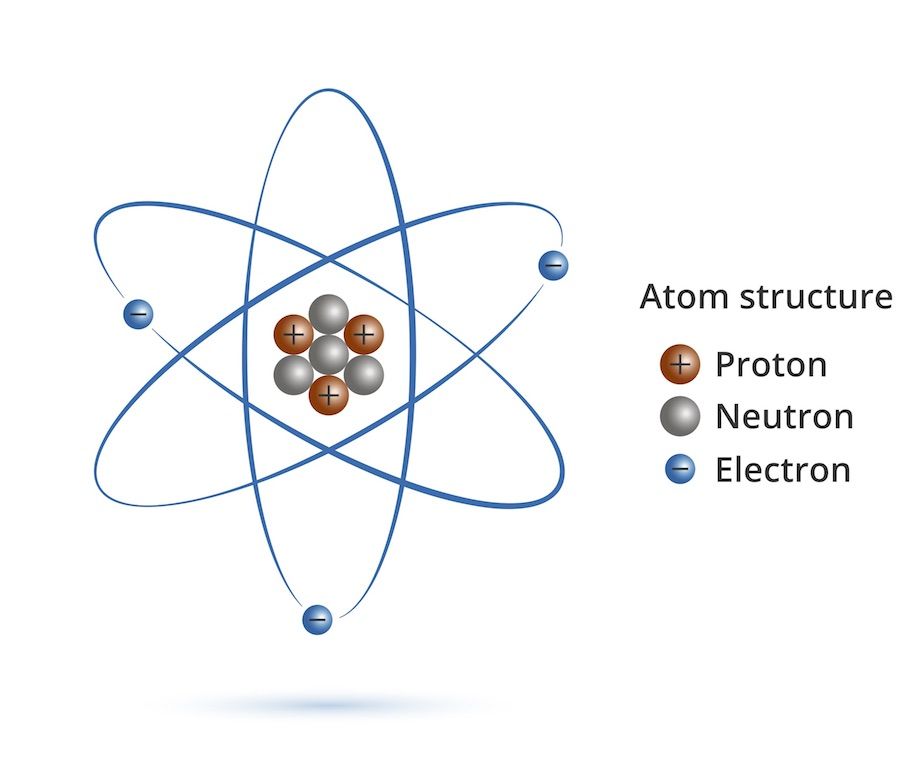
(2) Developing and Using Models. Neils Bohr’s model a nitrogen atom. Simple Model Of The Atom Every atom has a central, positively charged nucleus that is very small but massive The nucleus is made up of positive protons and neutral neutrons Electrons revolve around the nucleus in allowed orbits.
Sep 7, 18 - Explore LN Harrison's board "ATOM MODEL" on Pinterest. It still has its uses too;. The Bohr model works well for very simple atoms such as hydrogen (which has 1 electron) but not for more complex atoms.
These theories were later validated by observations made with the electron microscope. Soon after its proposal, the model was called a ' plum pudding ' model because the positive medium was like a pudding, with electrons, or plums, inside. We also know that atomic weight is a product of.
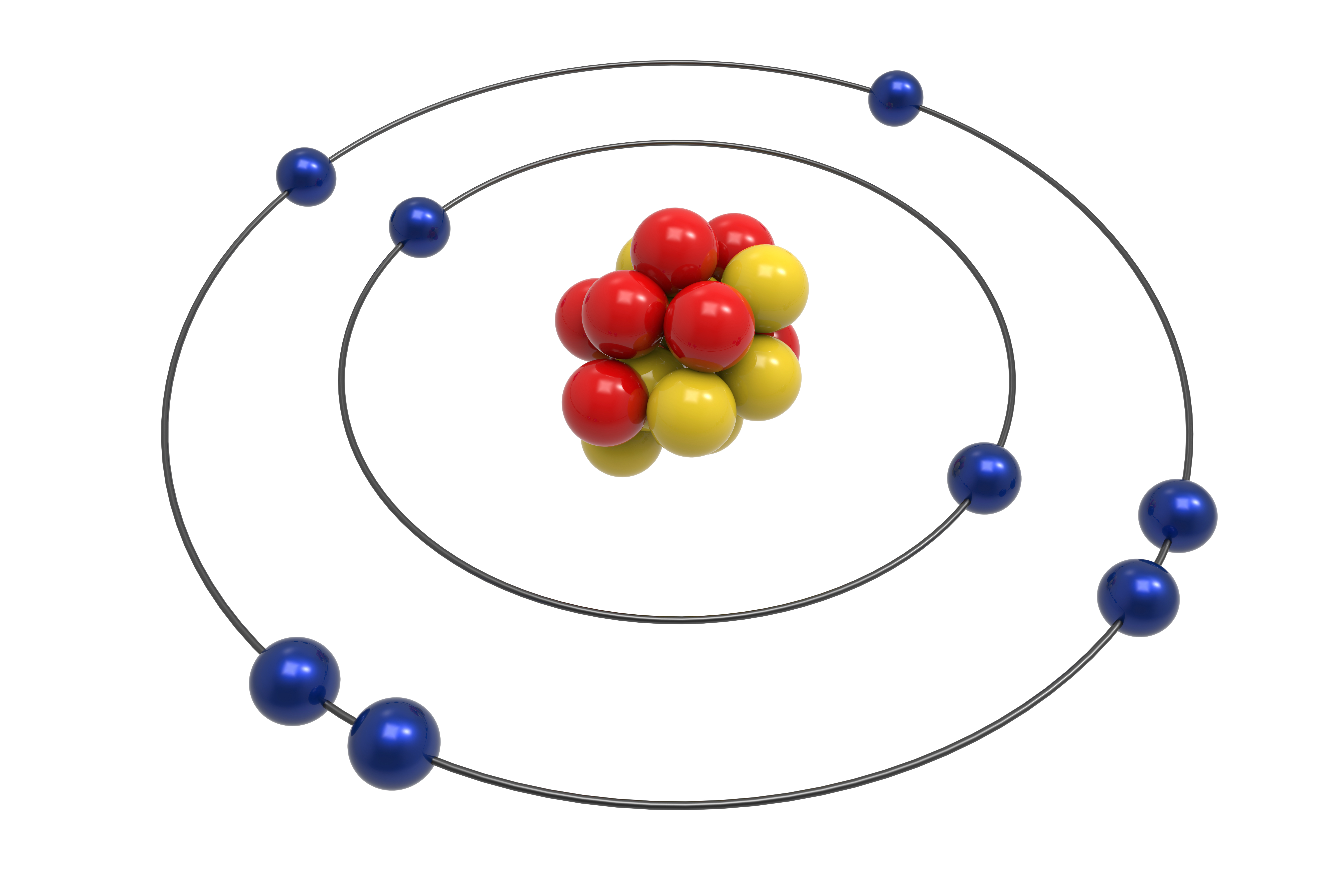
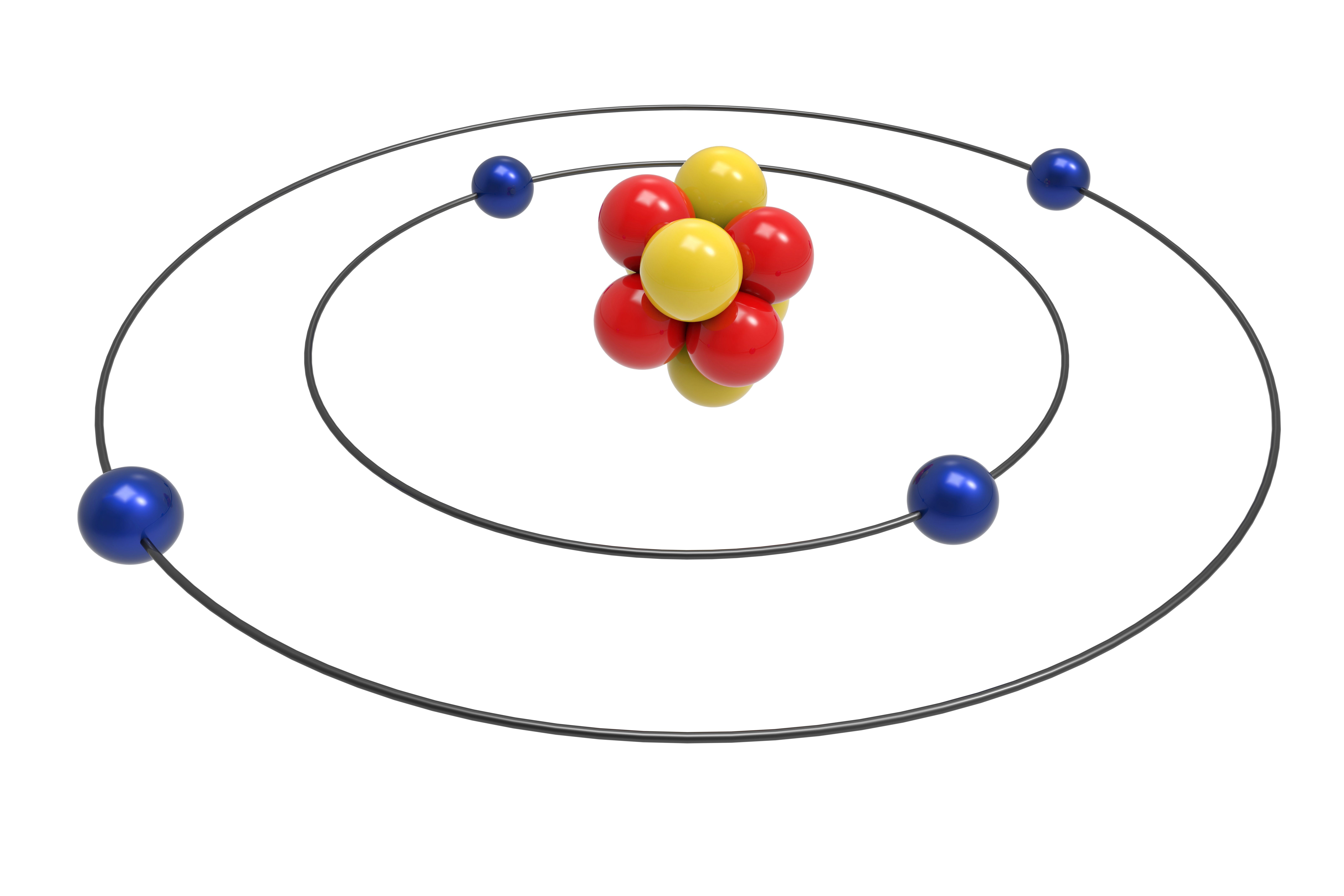
Electrons orbit the nucleus in orbits that have a set size and energy. An early model of the atom was developed by the physicist ErnestRutherford in 1912. The Bohr model and all of its successors describe the properties of.
With subtitles for english Merhaba arkadaşlar bu video atom modeli yapılışının ingilizce alt yazıl. Two models of atomic structure are in use today:. Model of the Atom An atom is a building block of matter that cannot be broken apart using any chemical means.
Niels Bohr proposed the Bohr Model of the Atom in 1915. Nuclear reactions can alter. Dalton's atomic model sets up the building blocks for others to improve on.
It needed slight modifications. Accepted scientific theory of atoms:. Basically, Schrödinger developed some pretty fancy math (called “wave functions”) to predict where the electrons in an atom are going to be found, 95% of the time.
The Bohr model and the quantum mechanical model. The following article will explain the timeline of the changing models of atom and the current model of the atomic structure. The Atomic Model of Matter Name_____ Block_____ Match the statement to the different atomic model.
Building Atomic Models {page 57}. OK let's make that model. The model is a way to help visualize the most probable position of electrons in an atom.
Rutherford directed the Geiger–Marsden experiment in 1909, which suggested, upon Rutherford's 1911 analysis, that J. Following the work of Ernest Rutherford and his colleagues in the early twentieth century, the picture of atoms consisting of tiny dense nuclei surrounded by lighter and even tinier electrons continually moving about the nucleus was well established. Atoms are extremely small and are made up of a few even smaller particles.
It takes a lot of atoms to make up anything. This video tells how to make a bohr atomic model. Apr 8, 16 - Explore The Homeschool Scientist's board "Atom Models", followed by people on Pinterest.
The Rutherford model was devised by the New Zealand-born physicist Ernest Rutherford to describe an atom. Everything in the world is made out of atoms. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time.
The students have started new specifications of OCR AS Chemistry A (H034),AQA AS Chemistry (7404), Edexcel AS Chemistry (8CH0) and CIE AS/A-level Chemistry (9701).All specifications require fundamental understanding of the changing models of atom and atomic structure. You can find the table in an encyclopedia, a science textbook or online. A simple model of the atom, symbols, relative atomic mass, electronic charge and isotopes study guide by Freddie_Gray5 includes 61 questions covering vocabulary, terms and more.
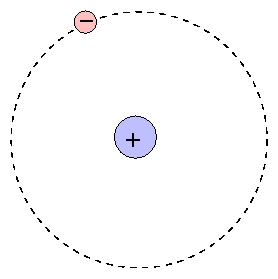
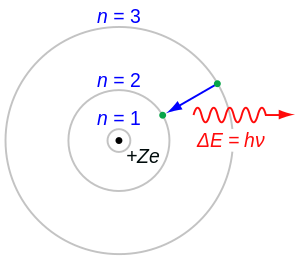
This picture was called the planetary model, since it pictured the atom as a miniature “solar system” with the electrons orbiting the nucleus. You will need 16 medium-sized polystyrene balls, a. According to Bohr's calculations for a hydrogen atom, the electron under normal conditions always stays at a certain distance from the nucleus.
_____ An atom is the smallest piece of matter. The scattering observed in Rutherford's experiments was soundly explained as the result of an atom with a dense (massive and small) core carrying a positive charge surrounded. •Avogadro also astutely reasoned that simple gases were not formed of solitary atoms but were instead compound molecules of two or more atoms.
Main Points of the Bohr Model. A simple classical model of an atom consists of a nucleus of a positive charge Ne surrounded by a spherical electron cloud of the same total negative charge. This week we’ll make a few more models with some Atomic Cookies (page 59).
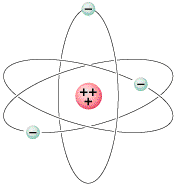
We begin with three simple assumptions:. At right is a simple atomic model of an atom. Protons and neutrons form.
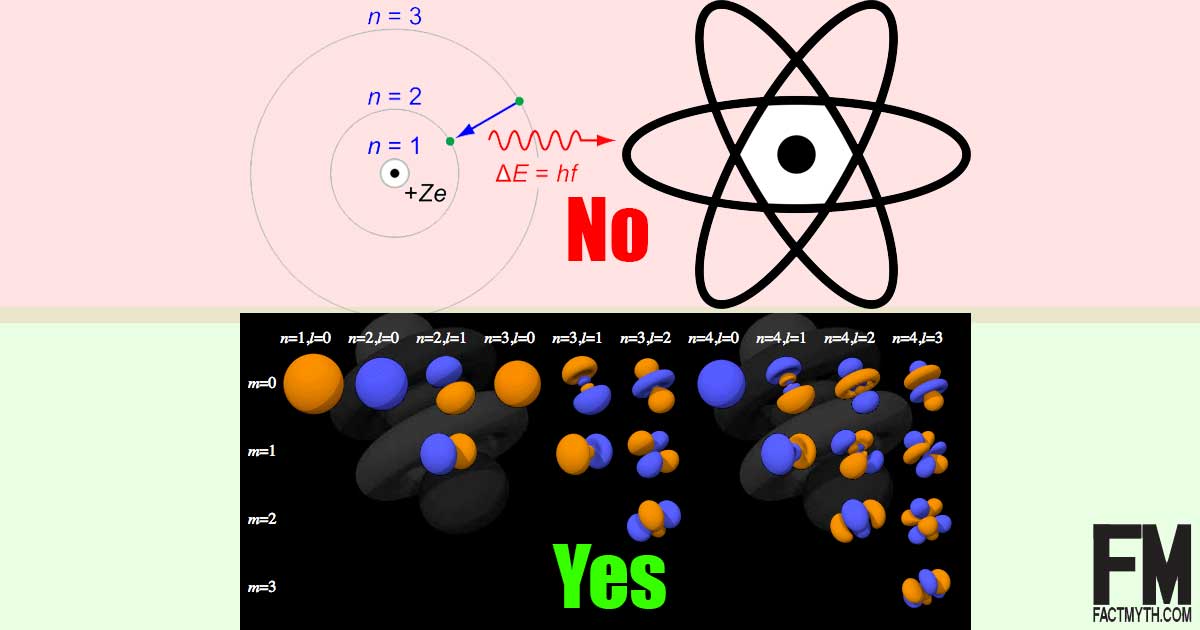
The atom for each model, and state the problem with each model. This lesson is based on California's Middle School Integrated Model of NGSS. Although the Bohr model is still used today, especially in elementary textbooks, a more sophisticated (and complex) model — the quantum mechanical model — is used much more frequently.
Bohr assumes that an electron in an atom is positioned at a specific detachment from the nucleus and is revolving round it with explicit velocity, i.e. Bohr Model of Hydrogen. In the so-called Rutherford atom, electrons orbit the nucleus in circular paths.
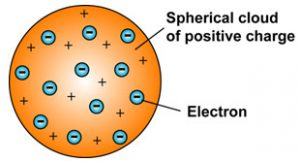
Thomson's model showed an atom that had a positively charged medium, or space, with negatively charged electrons inside the medium. (N is the atomicnumber and e is the magnitude of electronic charge.). The dried fruit (electrons) were stuck in a mass of pudding (protons).
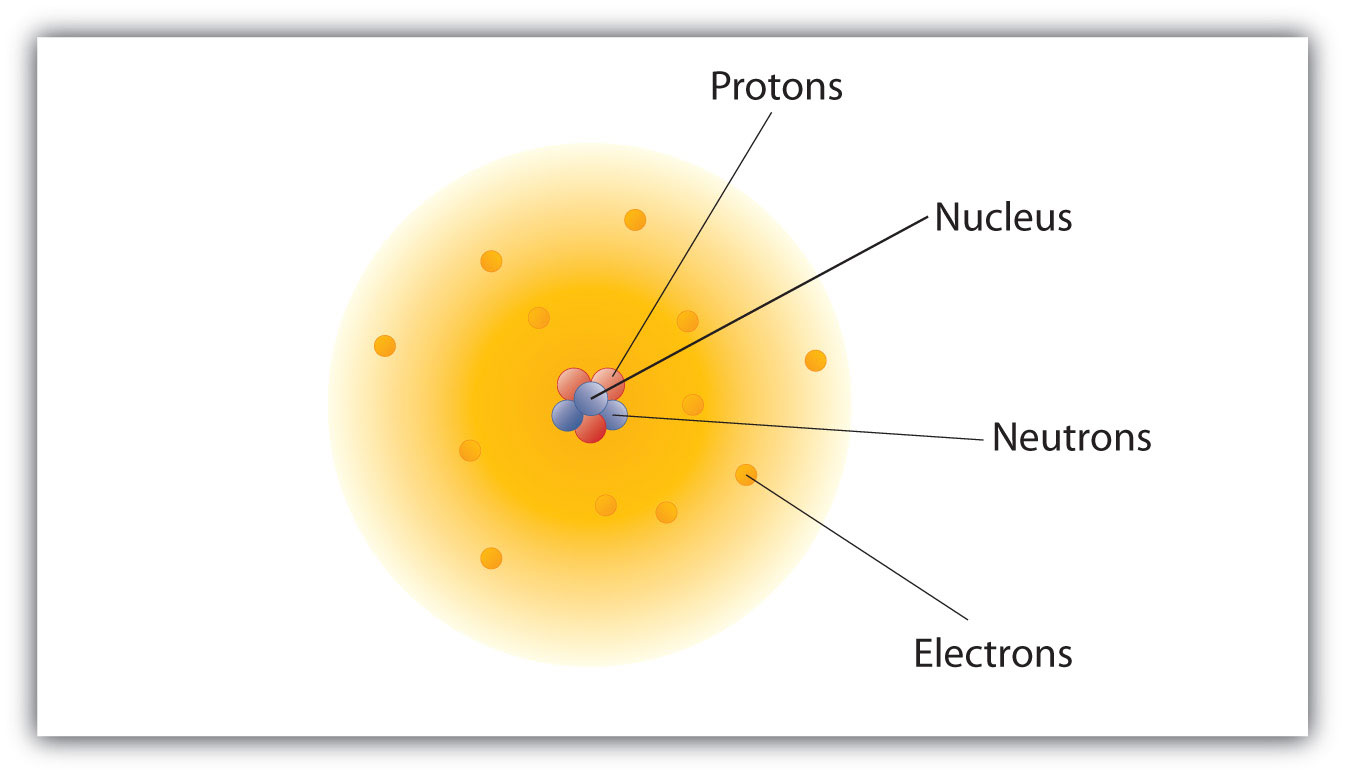
The nucleus is tiny compared to the atom as a whole:. To explain the two types of static electricity, he suggested that the. The plum pudding model After discovering the electron in 17, J J Thomson proposed that the atom looked like a plum pudding.
The quantum mechanical model is based on mathematics. In the very center of the atom is the nucleus. Simple Atomic Model All of the models have something valuable to offer in terms of visualizing matter at an atomic level.
Atoms fit together with other atoms to make up matter. However, it wasn't completely correct. Although it is more difficult to understand than the Bohr model, it can be used to explain observations made on complex atoms.
Atom Notes Element- pure substance that cannot be separated into simpler substances by physical or chemical means. The energy of the. All particles are wavelike, and an electron's wavelength <math>\lambda<math>, is related to its velocity v by:.
On the basis of the observations made during the experiment, Rutherford concluded that. Simple Model Diagrams For Hand He Atoms One Simple Model Of The Hydrogen Atom Pictures The H Atom As A Nucleus Of Charge +1 Surrounded By An Electron At Some Distance. James Chadwick and Discovery of Neutrons.
This nucleus is tiny and the rest of the atom is mostly empty space. Because the Bohr Model is a. In atomic physics, the Bohr model or Rutherford–Bohr model, presented by Niels Bohr and Ernest Rutherford in 1913, is a system consisting of a small, dense nucleus surrounded by orbiting electrons—similar to the structure of the Solar System, but with attraction provided by electrostatic forces in place of gravity.
Thomson, discovered that atoms contained tiny negatively charged particles called electrons. It’s quite handy for explaining chemical bonding and the reactivity of some groups of elements at a simple level. We then used their “Think About It” section to add a little fun to our Math Journal by drawing out an atom of lead.
The radius of an atom is about 0.1 nm (1 × 10-10 m);. Though some of his conclusions were incorrect, his contributions were vital. Which model does not able to explain the stability of an atom?.
He was the first to suggest that atoms are like miniature solar systems, except that the attractive force is not caused by gravity, but by opposing electrical charges. The Quantum Mechanical Model (p. Bohr’s model and the current model are described in Chapter 6, "The Structure of Atoms." Rutherford’s model of the atom is essentially the same as the modern model, except that it is now known that electrons are not uniformly distributed throughout an atom’s volume.
Everything in the world is made out of atoms. Therefore, the major part of an atom must be empty. Quizlet flashcards, activities and games help you improve your grades.
According to Goldstein’s experiment. This atomic model was discovered through the bombardment experiment of alpha particles on gold foil. In 1909, a scientist named Ernest Rutherford used the Geiger–Marsden experiment to prove that most of an atom is in a very small space called the atomic nucleus.
There were a lot of problems related to the calculation of the mass of the nucleus, although Rutherford’s model could explain the scattering of alpha. Bohr model, description of the structure of atoms, especially that of hydrogen, proposed (1913) by the Danish physicist Niels Bohr.The Bohr model of the atom, a radical departure from earlier, classical descriptions, was the first that incorporated quantum theory and was the predecessor of wholly quantum-mechanical models. Dalton's simple model of an atom persisted until 17, when another British physicist, J.J.
The basic particles that make up an atom are electrons, protons, and neutrons. The counting up to 125 was a lot of work!. Rutherford's model of an Atom was undoubtedly a breakthrough in Atomic studies.
See more ideas about Atom model, Atom, Atom project. This model, which is the basis of the modern understanding of the atom, is known as the quantum mechanical or wave mechanical model. Creating an Oxygen Atom Model.
None of the above Ans. Observations of Rutherford Model Experiment. Choose an atom with an atomic number of at least 11, since it has at least three energy level rings source:.
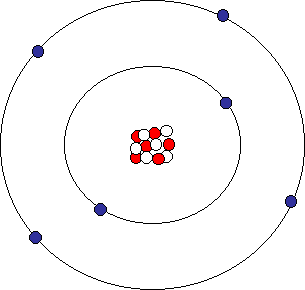
Sufficient electrons surround the nucleus. Structure and Properties of Matter - Substances are made from different types of atoms, which combine with one another in various ways. An atom is the basic unit of a chemical element.
The Bohr Model considers electrons to have both a known radius and orbit, which is impossible according to Heisenberg. Bohr Model of the Atom Explained Overview of the Bohr Model. At the time the words atom and molecule were used almost interchangeably.
1) De Broglie hypothesis:. Before you begin, look through the Periodic Table of Elements and pick an atom. Represented by a chemical symbol Atom – Smallest particle into which an element can be divided and still be the same substance.
The electron cloud model is currently the accepted model of an atom. Atoms are made of three main parts:. The radius of a nucleus (1 × 10-14 m) is less than \(\frac{1}{10,000.
See more ideas about Atom model, Atom, Atom project. The fact that there are only certain allowable states or energies that an electron in an atom can have is similar to a standing wave. Despite all this, Bohr’s is probably still the model of the atom you’re most familiar with, since it’s often the one first introduced during high school or secondary school chemistry courses.
He defined an atom as the smallest indivisible particle. Thomson 's plum pudding model of the atom was incorrect. If we take this model as being the true structure of atoms, it would answer a couple of big problems with existing traditional atomic models which assume that electrons orbit around the nucleus.
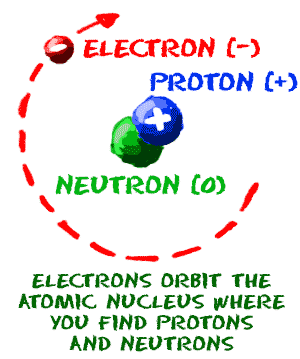
The atom is composed of a dense core that contains most of its mass and all of its positive charge, while low-mass negatively charged particles compose most of its volume. _____ Atoms are indivisible. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively.
Model Of A Hydrogen Atom Model Of A Helium Atom Electron The Electron "sees" A +1 Charge At The Nucleus The Electron "sees" A +2 Charge At The Nucleus Examining The Data In Table. The central region of an atom has a very small positively-charged nucleus which contains almost all the mass of the atom. How to Make a Small 3D Atom Model Method 1 of 3:.
You will need glue, scissors, cardstock, string,. Building a Calcium Atom Model. The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called electrons, circulate at some distance, much like planets revolving around the Sun.
The Bohr model is actually only accurate for one-electron systems such as the hydrogen atom or singly-ionized helium. Write the letter for the atomic model in front of the statement. The nucleus consists of positively charged protons, and neutrons, which have no charge.
Thomson created the plum pudding model, which stated that an atom was like plum pudding:.
Chem4kids Com Atoms Structure
What Are The 4 Atomic Models

A Simple One Dimensional Model Used To Introduce The Concept Of Download Scientific Diagram
Model Of An Atom Simple のギャラリー

How Many Quarks Would A Charm Quark Charm If A Charm Quark Could Charm Quarks Skulls In The Stars
Five Types Of Atomic Models
1

Atom Structure Model Atom Project For School Atom Project Making Youtube

Atom Wikipedia
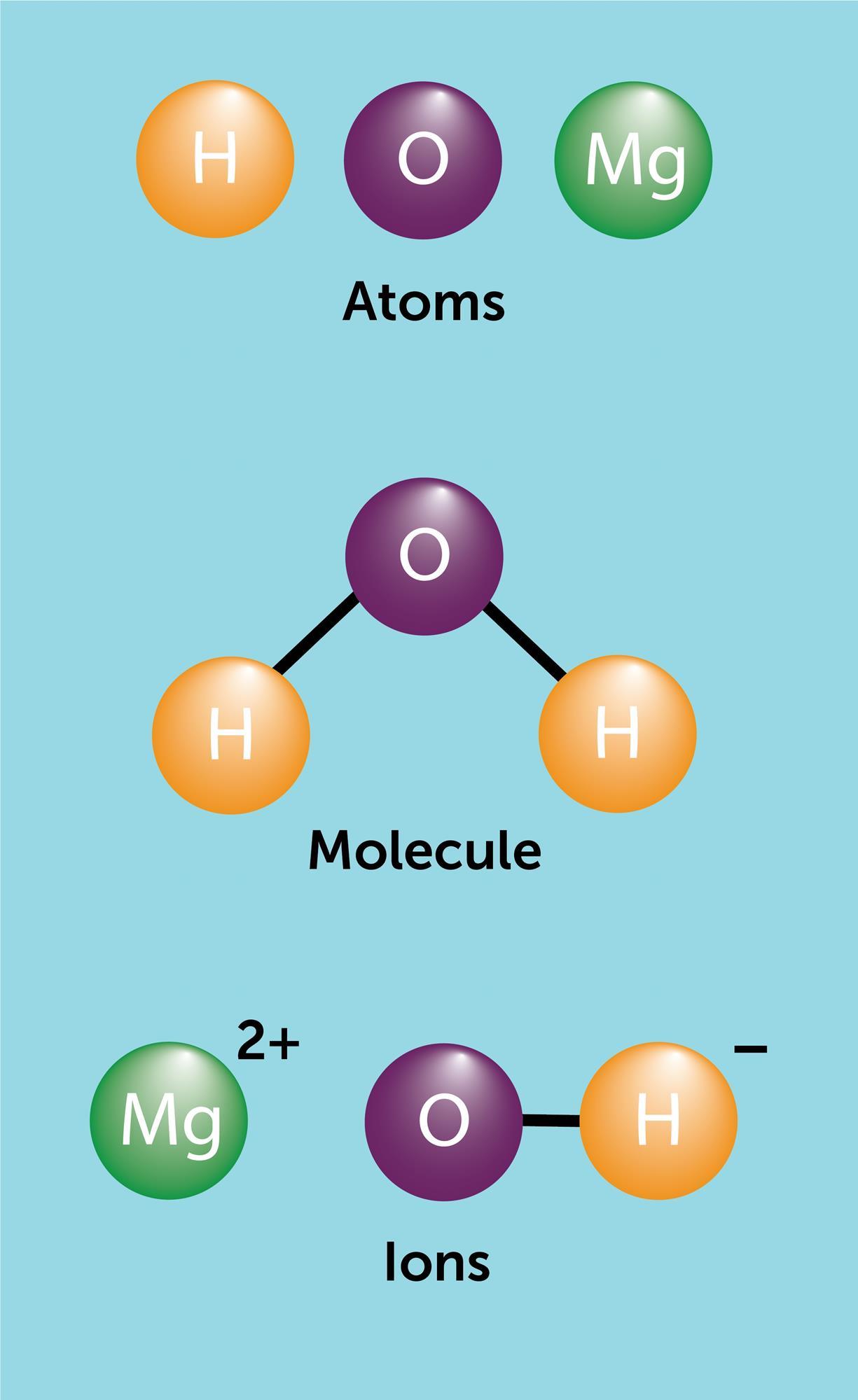
Atoms Molecules And Ions Cpd Rsc Education
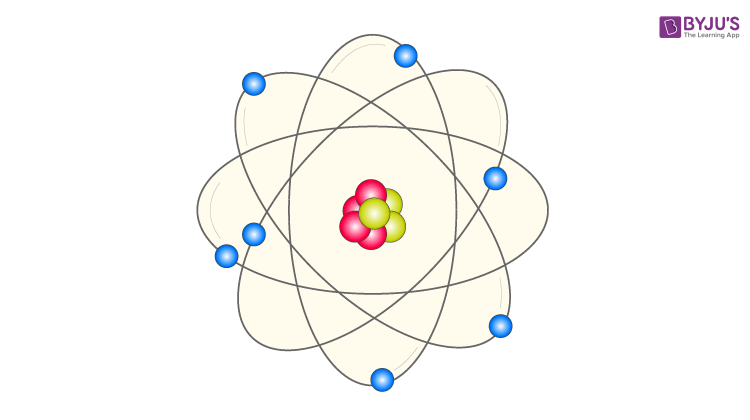
Bohr Model Of The Hydrogen Atom Equation Formula Limitations

Atomic Structure Electrons Protons Neutrons And Atomic Models

A Timeline Of Atomic Models Did You Know That The Atomic Model Has By Intlink Education Medium
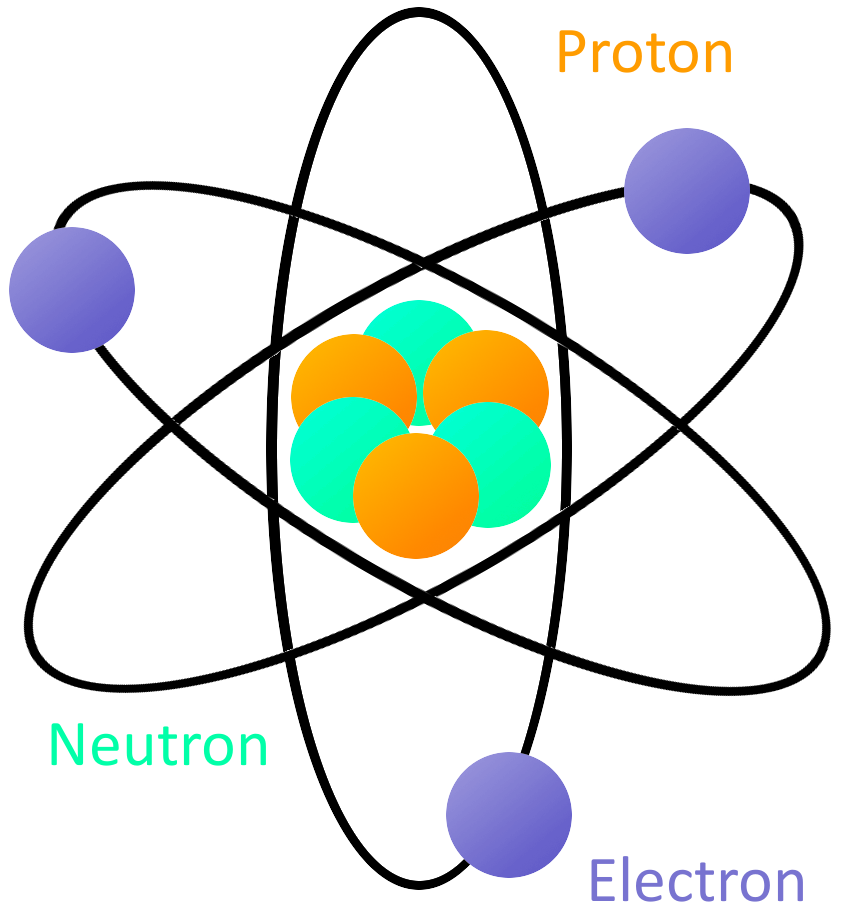
What Is Electricity Learn Sparkfun Com

Plum Pudding Model Of The Atom What Is It Who Discovered It Electrical4u
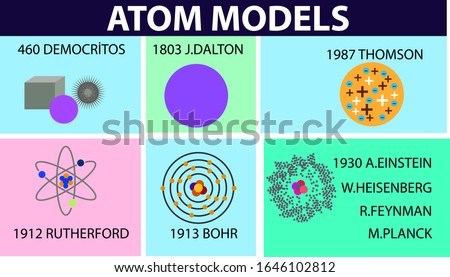
Shutterstock Puzzlepix
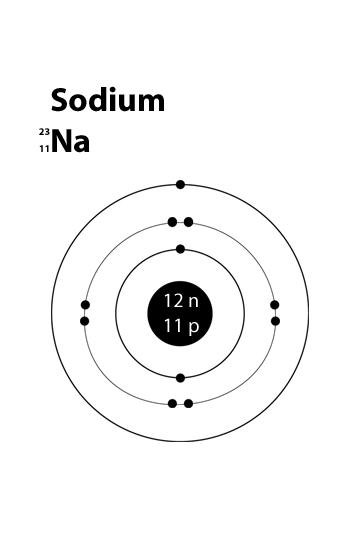
How Can I Make A Sodium Atom Model Socratic
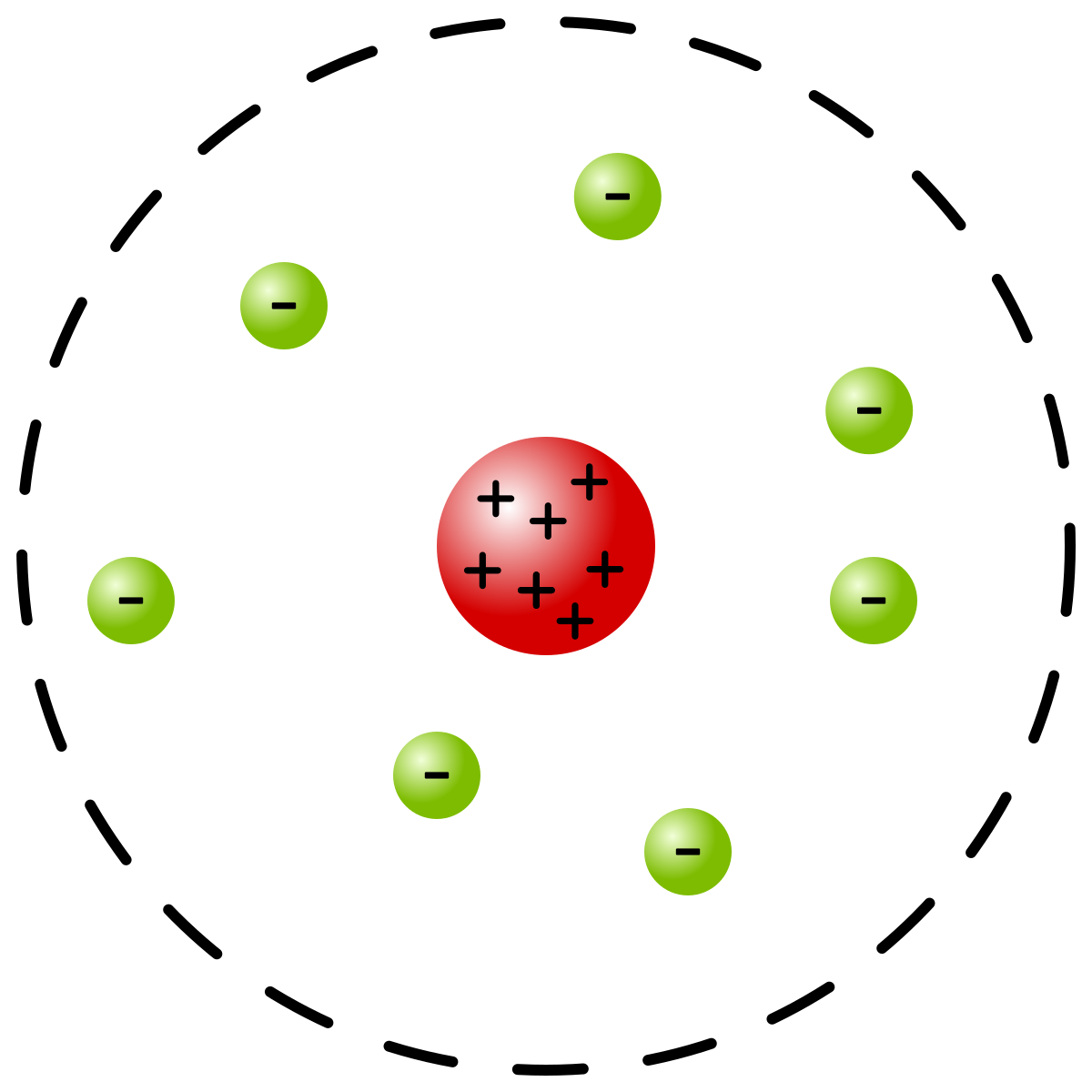
Rutherford Model Wikipedia

Basic Parts Of The Atom Protons Neutrons Electrons Nucleus Youtube
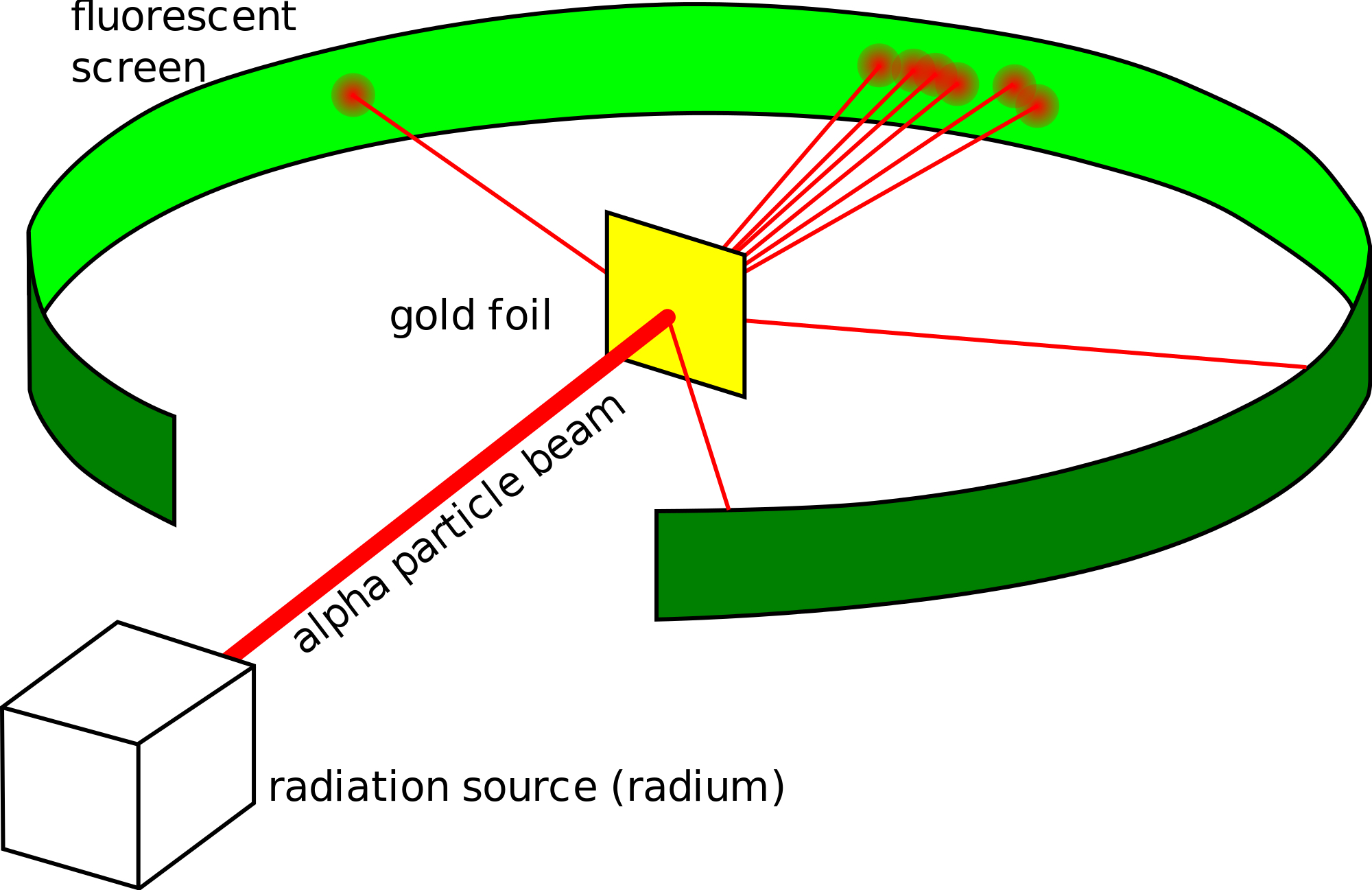
Experimental Evidence For The Structure Of The Atom

The Hydrogen Atom

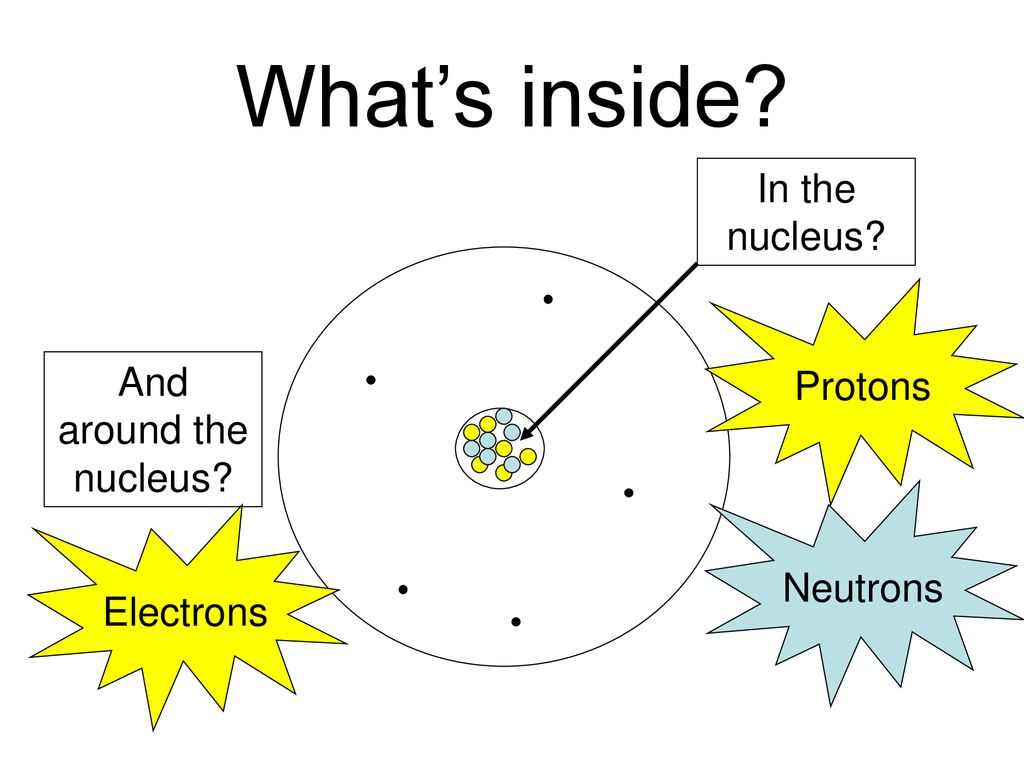
Chemical Ideas 2 1 A Simple Model Of The Atom What S Inside In The Nucleus Protons And Around The Nucleus Electrons Neutrons Ppt Download
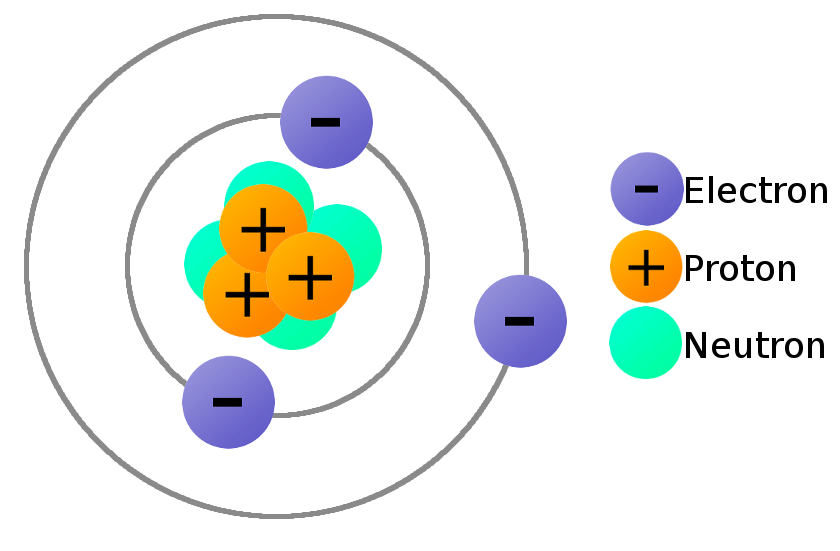
7 P 2a 1 Atomic Model

Rutherford Model Of The Atom Definition Diagram Video Lesson Transcript Study Com
Phyx 103 0 Atoms Heat

Atom Models
The Structure Of An Atom Explained With A Labeled Diagram Science Struck

Atomic Structure Teaching Resources The Science Teacher

The Structure Of The Atom Boundless Chemistry
The Bohr Model Is The Most Accurate Model Of An Atom Fact Or Myth
/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)
Basic Model Of The Atom Atomic Theory
The Boltzmann Equation

Rutherford Model Of The Atom Definition Diagram Video Lesson Transcript Study Com
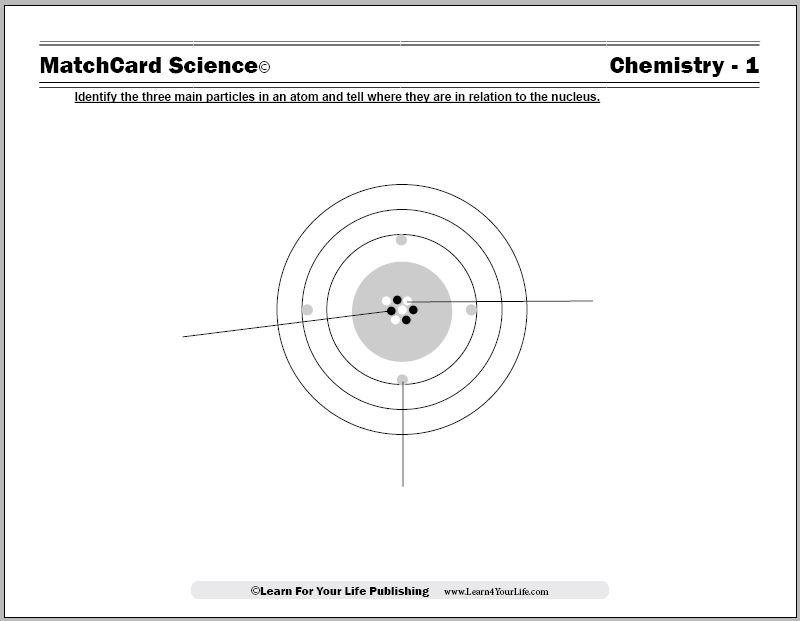
Model Of Atom

Simple Model Of The Atom Aqa Gcse Combined Science Questions Answers
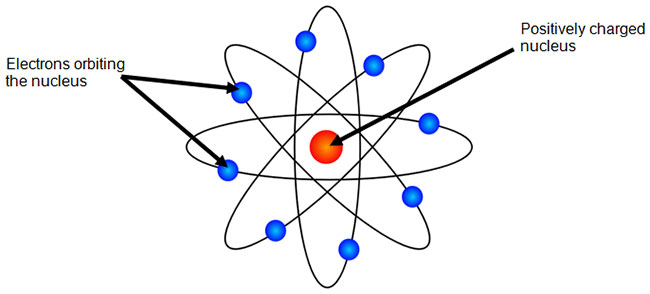
The Bohr Model Texas Gateway

Rutherford Atomic Model Electrical4u
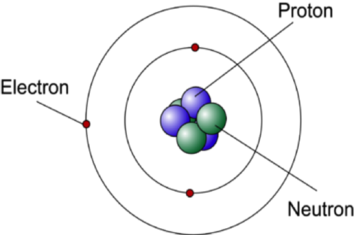
Bohr Model Study Material For Iit Jee Askiitians

1 1 A Simple Model Of The Atom Symbols Relative Atomic Mass Electronic Charge And Isotopes Pdf Atomic Nucleus Nuclear Chemistry
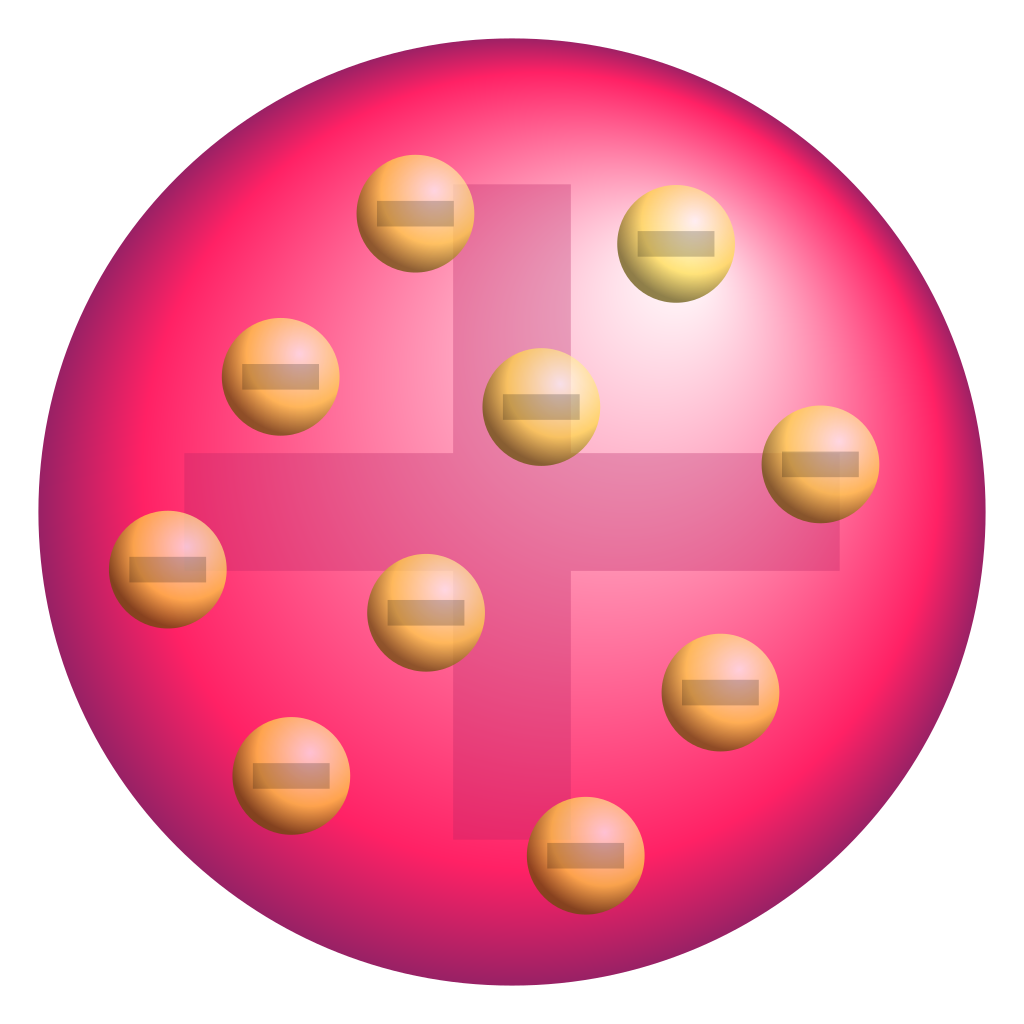
The Early Atom Boundless Physics
What Is An Atom Live Science

Atom Definition History Examples Britannica
Chem4kids Com Atoms Structure
.png?width=430&name=atom-1674878_640%20(1).png)
Understanding The Bohr Atomic Model

Simple Model Of The Atom Aqa Gcse Combined Science Questions Answers
Atomic Structure
Bohr Model Of The Atom Overview And Examples
Atomic Theory
What Is The Currently Accepted Model Of Atomic Structure Is There A More Recent Model Than The Bohr Or Bohr Sommerfeld That Has Been Observed By Physicists Quora

Q Tbn 3aand9gcrmlcfzprcu4fnzv1sskmhmeayk5haonja3ig Usqp Cau

3 Ways To Make A Small 3d Atom Model Wikihow
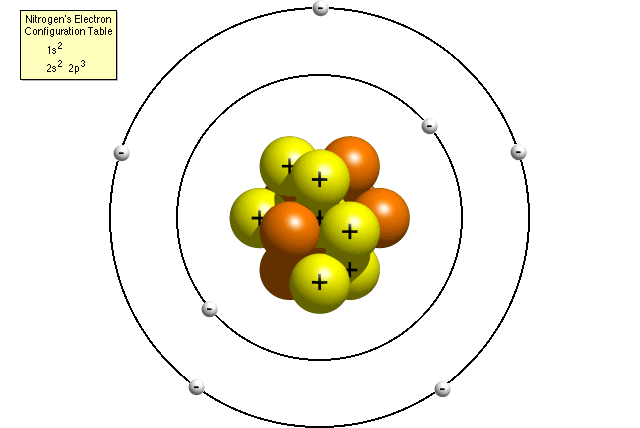
Questions And Answers How Do I Make A Model Of An Atom

Residue Side Chain Contact Model A All Atom Sidechain Model B Download Scientific Diagram

Atomic Structure The Changing Models Of Atom

What Is Bohr S Atomic Model Universe Today

The Structure Of An Atom Explained With A Labeled Diagram Science Struck
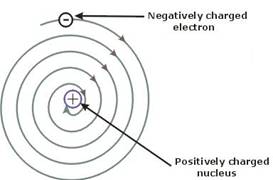
Write Drawbacks Of Rutherford S Atomic Model Chemistry Topperlearning Com Rl7qik3yy

A Simple Model Of The Atom Ppt Download

What Are The Parts Of An Atom Universe Today

How To Make A 3d Model Of An Atom Atom Model Atom Model Project Chemistry Projects
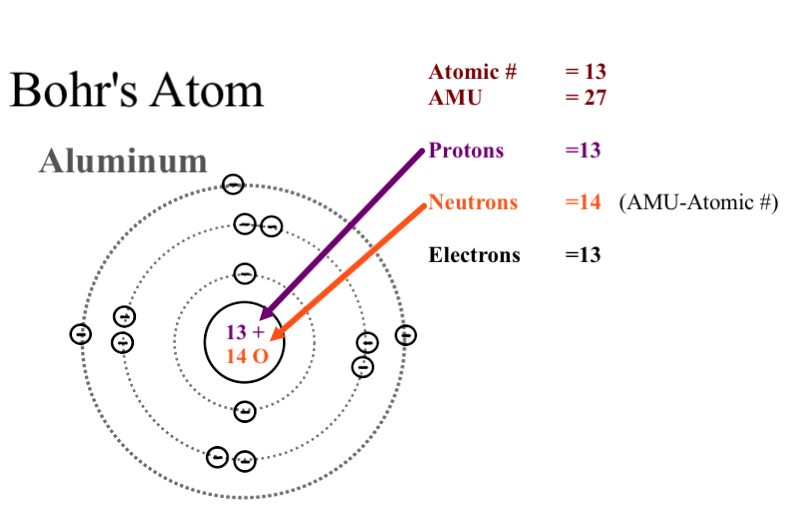
Why Could Bohr S Model Be Called A Planetary Model Of The Atom Socratic
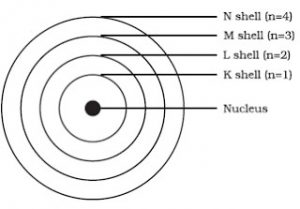
Bohr S Model Of An Atom Class 9 Structure Of An Atom
Bohr Model Of The Atom Overview And Examples
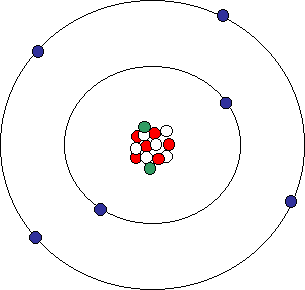
Atomic Structure

Thomson Atomic Model Description Image Britannica
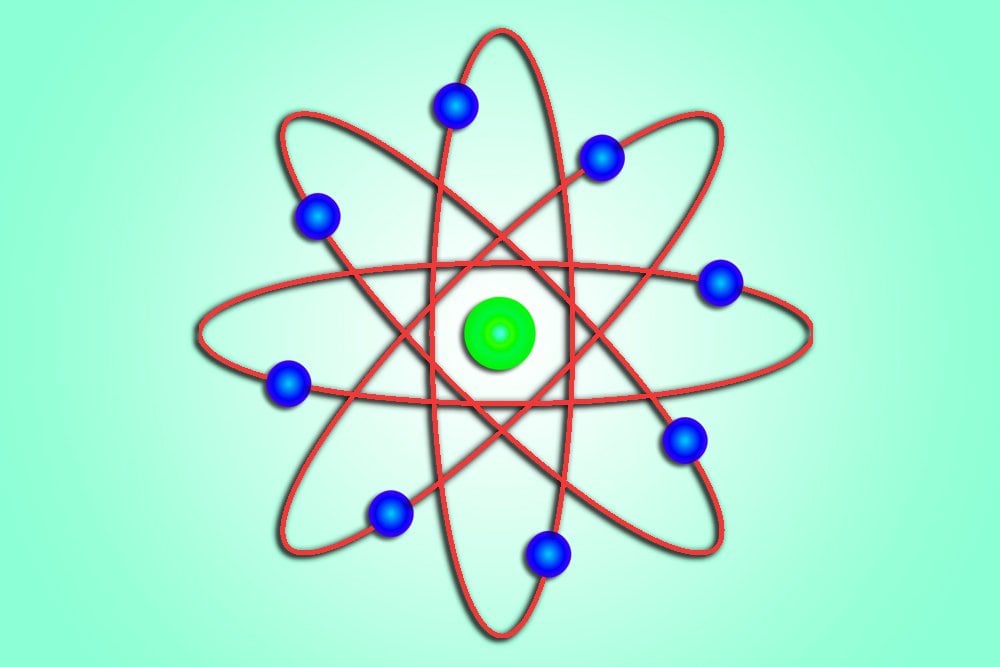
What Is The Dalton Atomic Model

5 Atom Models Atom Models Bohr Chemistry En Nuclear Plum Pudding Science Solid Sphere Wave Mechanical Glogster Edu Interactive Multimedia Posters
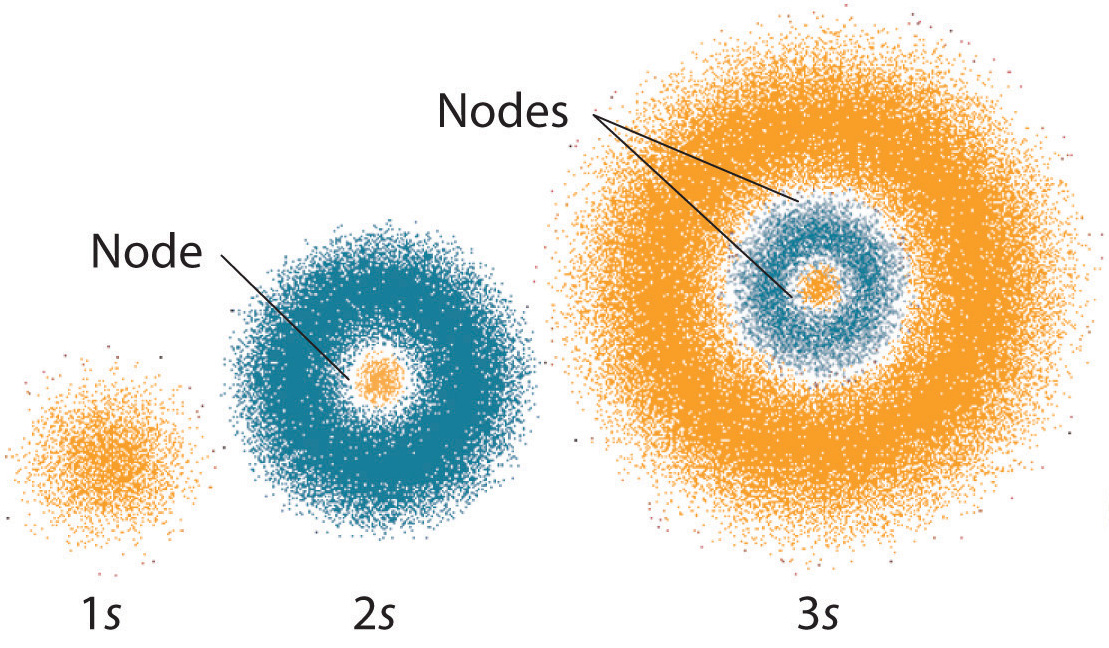
The Quantum Mechanical Model Of The Atom Article Khan Academy

Electron Cloud Atomic Model Ck 12 Foundation
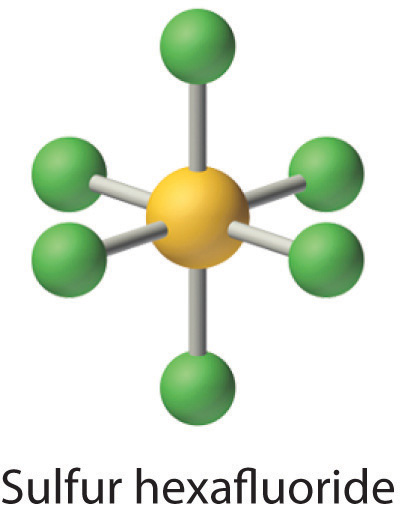
9 6 Exceptions To The Octet Rule Chemistry Libretexts

Bohr S Model For Hydrogen Atom

Inside The Atom Ck 12 Foundation
What Is The Dalton Atomic Model

This Is My Basic Simple Model Of The Atomic Structure It S An Atom With A Nucleus Containing Protons And Neutrons Atom Model Physical Science Projects Atom
Experimental Evidence For The Structure Of The Atom
Q Tbn 3aand9gcseyobbyyxbjn6tvzukgg8pqxexr4l9yuq G9cwemw2k9xszngu Usqp Cau
Q Tbn 3aand9gcta4pfzpyqnb4rzud2kzubv240xic7artbdpi Qxhlvh3neoyrf Usqp Cau

Introduction To Structure Of Atom Proton Neutron Electron With Examples
Atomic Number Wikipedia

A Timeline Of Atomic Models Did You Know That The Atomic Model Has By Intlink Education Medium

Simple Model Of An Atom Electrical Academia
What Are The Main And Most Important Postulates Of Bohr S Atomic Model Quora

What Is An Atom Live Science

Models Of The Atom The Atom Siyavula
J J Thomson Model Of An Atom Class 9 Structure Of An Atom

Elementary Particles

Atom Line Art 548 595 Transprent Png Free Download Line Art Angle Symmetry Cleanpng Kisspng

The Quantum Mechanical Model Of The Atom Article Khan Academy

Atoms Molecules E Chapter The Biology Primer

Hydrogen Atom Pictures Photos Images Of Chemistry Science For Kids Hydrogen Atom Molecule Model Science For Kids

4 1 1 A Simple Model Of The Atom Symbols Relative Atomic Mass Electronic Charge And Isotopes

Rutherford Model Of The Atom Definition Diagram Video Lesson Transcript Study Com
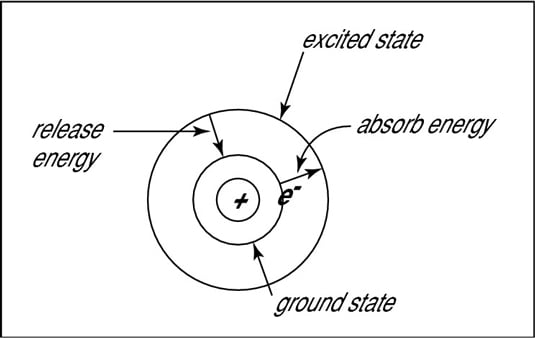
Atomic Structure The Bohr Model Dummies

Ways To Make An Atom Model Atom Model Atom Diagram Atom Model Project

The History Of The Atom Theories And Models Compound Interest
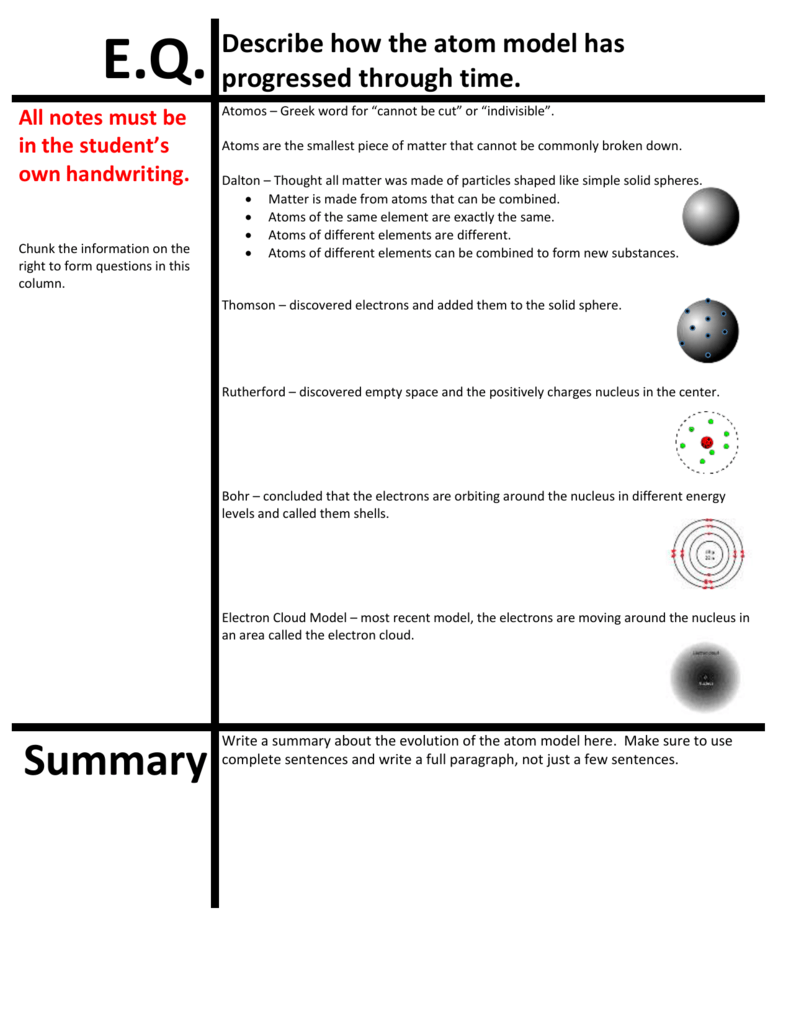
Evolution Of The Atom Model
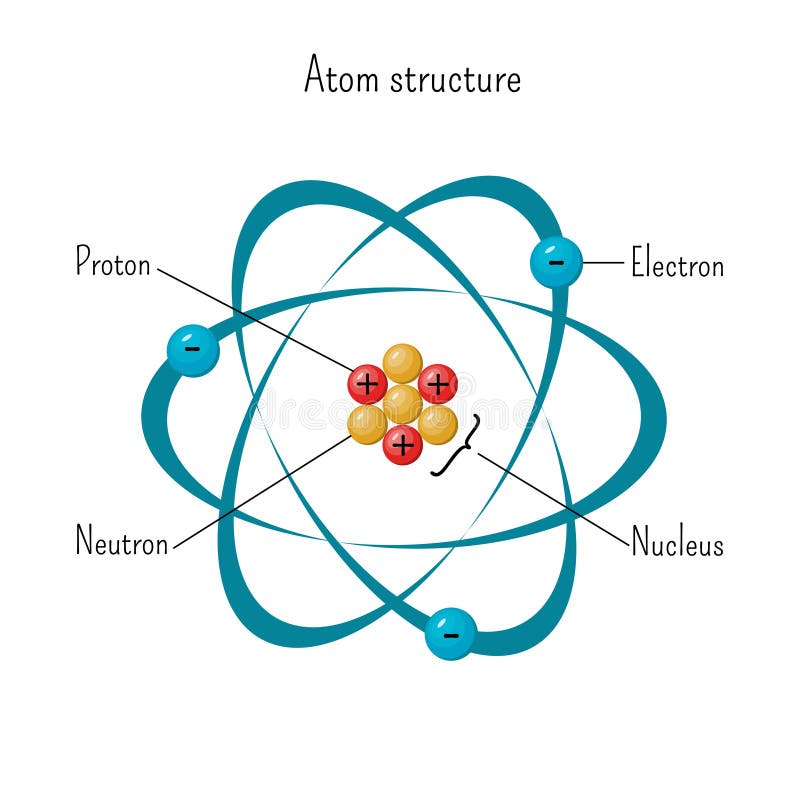
Simple Model Of Atom Structure With Electrons Orbiting Nucleus Of Three Protons And Neutrons Stock Vector Illustration Of Force Diagram
Q Tbn 3aand9gct Dl9qbx9tjh Soh7ux9ydwsgrjxwquyozpbdurjjszbpty0ux Usqp Cau
Atomic Models Wikilectures

5 Atom Models Atom Models Bohr Chemistry En Nuclear Plum Pudding Science Solid Sphere Wave Mechanical Glogster Edu Interactive Multimedia Posters

Non Electronics Teaching Aid m Ajis85

Rutherford Model Definition Facts Britannica

Bohr S Model Comer S Chemistry Classroom

History Of The Atom Ck 12 Foundation

Bohr S Model Of An Atom With Postulates And Limitations Byju S



